Professional Documents
Culture Documents
Boattrailers Coatings
Boattrailers Coatings
Uploaded by
aziz0 ratings0% found this document useful (0 votes)
5 views1 pageOriginal Title
boattrailers_coatings
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views1 pageBoattrailers Coatings
Boattrailers Coatings
Uploaded by
azizCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 1
Ask D. Gaw
Dear Dr. Galv: I’ve been doing boat trailers or hand
rails for a number of years and every once in a while
the coating will come out looking very rough. Why is
this happening and why does it happen in the center
section of the part ?
A. There are a couple of reasons that galvanizers
may find a very rough coating on a part such as a boat
trailer or a section of hand rail. The most obvious rea-
son is the fabricator used a different piece of steel for
one section of the assembly. The different piece of
steel had high levels of silicon or phosphorous and
was very reactive, causing the thick and rough coat-
ing. The rest of the assembly may be smooth and nor-
mal appearing galvanized coating. The inclusion of a
reactive piece of steel in the assembly is apparent
when the Yough coating stops abruptly at a weld line
or assembly joint, Sometimes itis ar attached bracket.
or clip ‘which has the rough coating. In the case of
hand fail, this rough coating may be the cause for
rejection of the assembly since the hand rail must be
smooth for people to run their hands along,
A second, more subtle reason for the rough coating
can be the steel chemistry of the steel tubing used to
fabricate the assembly. The fabricator will often have
specified a steel with a low silicon and phosphorous
content. The steel manufacturer will furnish the mill
certifications for the steel used to manufacture the
tubing and the chemistry will appear to be low reac-
tivity material as in the table below,
TABLET. TYPICAL Heat CHEMistty Fon TUBULAR STEEL
c Mn Pe Si Cu Sn Ni
0.10 0.33 0.010 0.006 0.025 005 0.003 0.025
The two reactive elements, phosphorous and silicon,
appear to be below the level where they cause thick
and rough coatings. When the rough coating appears,
Seger | Ocrorer FN Va\.37
some galvanizers are suspicious of the steel chem-
istry despite what the heat chemistry shows. The
galvanizers have taken samples of the actual steel
tubing used in the assembly and the results of one
example of this steel’s chemistry is shown in the
table below.
Fiance 2, ACTEAL Sree TUnNG CHEMISTRY
Cc Mn PS Si Gu Sh Ni
0.09 030 0.011 0.007 0.06 0.07 0.004 0.03,
‘The assembly with this steel chemistry showed
rough coatings on some of the pieces. The picture
shows the rough appearance of the coating. This
looks like the typical example of high phosphorous
steel but the actual reactive element is the silicon
The silicon level of 0.06 puts the steel on the leading |
edge of the Sandelin peak. Added factors such a5.
cold working the tubes to form the shape of the
boat trailer and the temperature cycle for the sec:
tion of tubing in the center of the assembly add to
the silicon reactivity. The silicon ison the edge of
the reactivity curve and will cause problems on
some pieces and not on others. ,This can be ver}
difficult to diagnose. é
‘These assemblies will provide adequate corrosion
protection but may be difficult to deliver to a very
particular customer because of the rough coating
appearance. As a galvanizer, there is very little
that can be done about this rough appearance
because the problem is caused by the steel chem-
istry. Steel heat chemistries are an average of
sample pieces and do not always reflect the
actual chemistry of the tubing parts.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5819)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (845)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- A Tracker Instrument 2015 ENDocument11 pagesA Tracker Instrument 2015 ENazizNo ratings yet
- Wet DrykettleDocument1 pageWet DrykettleazizNo ratings yet
- Grey CoatingDocument1 pageGrey CoatingazizNo ratings yet
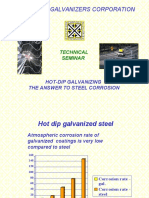
- Galvanized Coatings SeminarDocument27 pagesGalvanized Coatings Seminaraziz50% (2)
- Gal Rebar SeminarDocument11 pagesGal Rebar SeminarazizNo ratings yet
- To: Our Value Customer: Galvaco Industries SDN Bhd.Document1 pageTo: Our Value Customer: Galvaco Industries SDN Bhd.azizNo ratings yet