Professional Documents
Culture Documents
A Brief History and Evolution of Pharmaceutical Laws in India
Uploaded by
Tushar SaxenaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Brief History and Evolution of Pharmaceutical Laws in India
Uploaded by
Tushar SaxenaCopyright:
Available Formats
A brief history and evolution of pharmaceutical laws in India
The history of medical science is as old as humans itself, at the beginning the mankind learned from
the basic instincts, from the observation of birds and animals. They used cold water, leaves, dirt etc.
for soothing. Slowly these methods developed and became the roots of modern medication and
pharmacy.
In ancient India also, people practised a very developed medical system. The earliest information on
about medicine and medical science is available from Vedic period with four Vedas along with their
Brahmanas, Aranyakas, and Upanishads.
Later, the medical observations from the Vedic period laid the foundation for a more rational and
methodical system known as Ayurveda.
Nevertheless, this system of medicine declined when Muslim invaded India, and with them, the
Arabic or the Unani-Tibbi system flourished. However, until now, there were no scientific methods of
standardisation of drugs.
The medical system in India saw another turn with British rule. The allopathic system became
prominent, and initially, all drugs were imported from Europe. Later some drugs began to be
manufactured in this country.
The Britishers at first imposed some laws having a direct or indirect bearing on drugs but they were
insufficient-
1878 Opium Act- The manufacturing, cultivation, export-import and sale of opium were dealt
with this act.
1889 Indian Merchandise Act- Misbranding of goods in general
1894 Indian Tariff Act- Imposing customs duty on goods including foods, drugs, chemicals and
medicines imported into India or exported therefrom.
1898 Sea Customs Act -Goods with ‘false trade description’ were prevented from importing
under this act.
1919 Poisons Act Regulated the import, possession and sale of poisons.
Indian Penal Code - Some sections deal with adulteration as a punishable offence.
Dawn of Pharmaceutical Profession and laws in India
On 11th August 1930 government of India appointed a committee under the chairmanship of Late
Col. R.N. Chopra. The purpose of the committee was to see problems of Pharmacy in India and
recommend the measures.
The committee published its report in 1931. According to it, there was no recognised profession of
Pharmacy in India. Only a few compounders were filling the gap.
After that, Prof. M.L. Schroff initiated university level pharmaceutical education at Banaras Hindu
University. In 1935 the United Province Pharmaceutical Association was established which later
became Indian Pharmaceutical Association.
The government of India brought ‘Import on Drugs Bill’ in 1937; however, it was later withdrawn. In
1940 the government brought ‘Drugs Bill’ which was later adopted as Drugs and cosmetics act 1940.
This act aimed to regulate import, manufacture, sale and distribution of drugs in India.
Next year under this act, the Drugs Technical Advisory Board was constituted, and Central Drugs
Laboratory was established in Calcutta. In 1945 the government established Drugs rule under Drugs
act 1940.
In 1945 the government brought pharmacy bill to standardise the pharmacy education in India, and in
1948, the Pharmacy Act was adopted.
In 1948 Indian Pharmaceutical Committee was established under the chairmanship of Dr B.N. Ghosh.
In 1949 Pharmacy Council of India was established under the Pharmacy Act.
The Drugs and Magic Remedies (Objectionable Advertisement) Act was brought in 1954. The object of
this act was to control the advertisement regarding drugs. The act prohibits the advertising of drugs
claiming to have magical qualities.
The Narcotic Drugs and Psychotropic Substances Act was enacted in 1985 to protect society from the
danger of addictive drugs. It controls and regulates the operations relating to Narcotic Drugs and
Psychotropic Substances.
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- MemorialDocument25 pagesMemorialAnubhuti Shreya62% (13)
- SPOT X DevDocument52 pagesSPOT X DevJaoNo ratings yet
- Moot Court MemorialDocument22 pagesMoot Court MemorialAnubhuti Shreya81% (57)
- UFO FILES Black Box Ufo SecretsDocument10 pagesUFO FILES Black Box Ufo SecretsNomad XNo ratings yet
- Adverse Effects of Artificial Food Colouring On ChildrenDocument2 pagesAdverse Effects of Artificial Food Colouring On ChildrenTushar SaxenaNo ratings yet
- Detailing Studio's Ceramic Coating A Guardian For Your MaseratiDocument1 pageDetailing Studio's Ceramic Coating A Guardian For Your MaseratiTushar SaxenaNo ratings yet
- Our Passion Is To Protect Your BuickDocument1 pageOur Passion Is To Protect Your BuickTushar SaxenaNo ratings yet
- Our Goal Is Your Bentley's ProtectionDocument1 pageOur Goal Is Your Bentley's ProtectionTushar SaxenaNo ratings yet
- Reinvent Your Maruti With Detailing Studio's Ceramic CoatingDocument1 pageReinvent Your Maruti With Detailing Studio's Ceramic CoatingTushar SaxenaNo ratings yet
- Drug Abuse Epidemic in IndiaDocument10 pagesDrug Abuse Epidemic in IndiaPushpendra SinghNo ratings yet
- Major Food Safety Laws in IndiaDocument3 pagesMajor Food Safety Laws in IndiaTushar SaxenaNo ratings yet
- Food Security Challenges India Faced During The LockdownDocument2 pagesFood Security Challenges India Faced During The LockdownTushar SaxenaNo ratings yet
- Detailing Studio's Ceramic Coating The Ultimate Protection For Your CadillacDocument1 pageDetailing Studio's Ceramic Coating The Ultimate Protection For Your CadillacTushar SaxenaNo ratings yet
- Oxytocin's Harmful Effects on Animals and HumansDocument1 pageOxytocin's Harmful Effects on Animals and HumansTushar SaxenaNo ratings yet
- Technology & BankingDocument15 pagesTechnology & Bankingswati100491% (11)
- Health Risks of Pesticide Residue in Our FoodDocument2 pagesHealth Risks of Pesticide Residue in Our FoodTushar SaxenaNo ratings yet
- PS5 Release DateDocument1 pagePS5 Release DateTushar SaxenaNo ratings yet
- Major Food Safety Laws in IndiaDocument3 pagesMajor Food Safety Laws in IndiaTushar SaxenaNo ratings yet
- Dell LaptopsDocument2 pagesDell LaptopsTushar SaxenaNo ratings yet
- Boeing and Porche Work On A Flying CarDocument1 pageBoeing and Porche Work On A Flying CarTushar SaxenaNo ratings yet
- Teaching NotesDocument12 pagesTeaching NotesTushar SaxenaNo ratings yet
- Dive Into LondonDocument1 pageDive Into LondonTushar SaxenaNo ratings yet
- Blizzard Reduces The Ban Imposed On PlayerDocument1 pageBlizzard Reduces The Ban Imposed On PlayerTushar SaxenaNo ratings yet
- Chrome Will Come With Password CheckupDocument2 pagesChrome Will Come With Password CheckupTushar SaxenaNo ratings yet
- AMD Will Reportedly Launch Next Gen SoonDocument1 pageAMD Will Reportedly Launch Next Gen SoonTushar SaxenaNo ratings yet
- Understand competition in any industry using Porter's 5 Forces ModelDocument5 pagesUnderstand competition in any industry using Porter's 5 Forces ModelTushar SaxenaNo ratings yet
- Apps Supported by Smart Speaker Can SpyDocument1 pageApps Supported by Smart Speaker Can SpyTushar SaxenaNo ratings yet
- What Is Banking Ombudsman?Document5 pagesWhat Is Banking Ombudsman?Tushar SaxenaNo ratings yet
- Ronda Rousey To Start Streaming Games in FacebookDocument1 pageRonda Rousey To Start Streaming Games in FacebookTushar SaxenaNo ratings yet
- Boeing and Porche Work On A Flying CarDocument1 pageBoeing and Porche Work On A Flying CarTushar SaxenaNo ratings yet
- Legal HistoryDocument15 pagesLegal HistoryTushar SaxenaNo ratings yet
- USP-NF 1251 Weighing On An Analytical BalanceDocument7 pagesUSP-NF 1251 Weighing On An Analytical BalanceZerish InaayaNo ratings yet
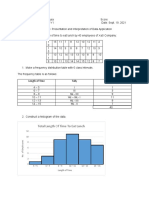
- Cedrix James Estoquia - OLLC Lesson 4.6 Presentation and Interpretation of Data ApplicationDocument4 pagesCedrix James Estoquia - OLLC Lesson 4.6 Presentation and Interpretation of Data ApplicationDeuK WR100% (1)
- Power Electronics Lab 1 (07DEM19F1005)Document15 pagesPower Electronics Lab 1 (07DEM19F1005)Mohd Faizul Idham AhmadNo ratings yet
- The Power ParadoxDocument27 pagesThe Power ParadoxKieran De PaulNo ratings yet
- ApplicationDocument11 pagesApplicationKamal Ra JNo ratings yet
- 10.6 Heat Conduction Through Composite WallsDocument35 pages10.6 Heat Conduction Through Composite WallsEngr Muhammad AqibNo ratings yet
- Autochief® C20: Engine Control and Monitoring For General ElectricDocument2 pagesAutochief® C20: Engine Control and Monitoring For General ElectricВадим ГаранNo ratings yet
- Time: 3 Hours Total Marks: 100: Printed Page 1 of 2 Sub Code: KEE302Document2 pagesTime: 3 Hours Total Marks: 100: Printed Page 1 of 2 Sub Code: KEE302AvinäshShärmaNo ratings yet
- DIK 18A Intan Suhariani 218112100 CJR Research MethodologyDocument9 pagesDIK 18A Intan Suhariani 218112100 CJR Research MethodologyFadli RafiNo ratings yet
- Liquefaction of Soil in KathmanduDocument9 pagesLiquefaction of Soil in Kathmanduajay shresthaNo ratings yet
- Netprobe 2000Document351 pagesNetprobe 2000Jordon CampbellNo ratings yet
- Investigação de Um Novo Circuito de Balanceamento de Tensão para Turbinas Eólicas Baseadas em PMSG Offshore Conectadas em ParaleloDocument7 pagesInvestigação de Um Novo Circuito de Balanceamento de Tensão para Turbinas Eólicas Baseadas em PMSG Offshore Conectadas em ParaleloDaniel reisNo ratings yet
- Short Time Fourier TransformDocument37 pagesShort Time Fourier TransformGopikaPrasadNo ratings yet
- SARTIKA LESTARI PCR COVID-19 POSITIVEDocument1 pageSARTIKA LESTARI PCR COVID-19 POSITIVEsartika lestariNo ratings yet
- Homework FileDocument18 pagesHomework FiledariadybaNo ratings yet
- Work Text INGE09: Understanding The SelfDocument24 pagesWork Text INGE09: Understanding The SelfFrancis Dave MabborangNo ratings yet
- Assignment 7Document1 pageAssignment 7sujit kcNo ratings yet
- CBSE KV Class VIII SA I Maths Sample Question Paper 2015Document3 pagesCBSE KV Class VIII SA I Maths Sample Question Paper 2015Amrita SenNo ratings yet
- General Appraiser Market Analysis and Highest & Best Use-Schedule PDFDocument7 pagesGeneral Appraiser Market Analysis and Highest & Best Use-Schedule PDFadonisghlNo ratings yet
- Comprehensive Exam SchedDocument16 pagesComprehensive Exam SchedMark ErvinNo ratings yet
- Modern Tips For The Modern Witch (/)Document5 pagesModern Tips For The Modern Witch (/)Rori sNo ratings yet
- Tecnair Close Control Catalog 0213Document36 pagesTecnair Close Control Catalog 0213Uzman HassanNo ratings yet
- Full Download Ebook Ebook PDF Oracle 12c SQL 3rd Edition by Joan Casteel PDFDocument41 pagesFull Download Ebook Ebook PDF Oracle 12c SQL 3rd Edition by Joan Casteel PDFdaniel.morones654100% (36)
- Government of India Sponsored Study on Small Industries Potential in Sultanpur DistrictDocument186 pagesGovernment of India Sponsored Study on Small Industries Potential in Sultanpur DistrictNisar Ahmed Ansari100% (1)
- ST5DB Skylake U Platform Block Diagram TitleDocument82 pagesST5DB Skylake U Platform Block Diagram TitleJOSE PAZNo ratings yet
- Mil PRF 23699FDocument20 pagesMil PRF 23699FalejandroNo ratings yet
- PricelistDocument2,276 pagesPricelistadilcmsNo ratings yet
- Strategic ManagementDocument7 pagesStrategic ManagementSarah ShehataNo ratings yet