Professional Documents
Culture Documents
English Description Covid-19
Uploaded by
Francisco Javier Cerezo Lacasa0 ratings0% found this document useful (0 votes)
22 views2 pagesOriginal Title
English description Covid-19
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
22 views2 pagesEnglish Description Covid-19
Uploaded by
Francisco Javier Cerezo LacasaCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
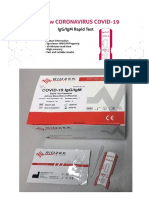
MSLRDT1 00 IgG/IgM Rapid Test For in vitro diagnostic use only.
For healthcare professionals and professionals at point of care sites.
Cassette (WB/S/P)
Do not use after the expiration date.
English
Please read all the information in this leaflet before performing the test.
For professional and in vitro diagnostic use only. The test cassette should remain in the sealed pouch until use.
[INTENDED USE] All specimens should be considered potentially hazardous and handled
in the same manner as an infectious agent.
The MSLRDT100 IgG/IgM Rapid Test Cassette is a lateral flow The used test cassette should be discarded according to federal, state
chromatographic immunoassay for the qualitative detection of antibodies and local regulations.
(IgG and IgM) to Novel coronavirus in human Whole Blood/Serum/Plasma.
[COMPOSITION]
It provides an aid in the diagnosis of infection with Novel coronavirus. (The picture is for reference only, please refer to the material object.)
The test contains a membrane strip coated with Mouse anti-Human IgM
[SUMMARY] antibody and Mouse anti-Human IgG antibody on the test line, and a dye [INTERPRETATION OF RESULTS]
Early January 2020, a novel coronavirus (SARS-CoV-2, formerly known as pad which contains colloidal gold coupled with Novel coronavirus Positive: Control line and at least one test line appear on the membrane.
2019-nCoV) was identified as the infectious agent causing an outbreak of recombinant antigen. The appearance of IgG test line indicates the presence of Novel
viral pneumonia in Wuhan, China, where the first cases had their symptom The quantity of tests was printed on the labeling. coronavirus specific IgG antibodies. The appearance of IgM test line
onset in December 2019. Materials Provided indicates the presence of Novel coronavirus specific IgM antibodies. And if
Coronaviruses are enveloped RNA viruses that are distributed broadly Test cassette Package insert both IgG and IgM line appear, it indicates that the presence of both Novel
among humans, other mammals, and birds and that cause respiratory, Buffer coronavirus specific IgG and IgM antibodies.
enteric, hepatic, and neurologic diseases.Six coronavirus species are Materials Required But Not Provided Negative: One colored line appears in the control region(C).No apparent
known to cause human disease. Four viruses-229E, OC43, NL63, and Specimen collection container Timer colored line appears in the test line region.
HKU1 are prevalent and typically cause common cold symptoms in Invalid: Control line fails to appear. Insufficient specimen volume or
immunocompetent individuals. The two other strains severe acute
[STORAGE AND STABILITY] incorrect procedural techniques are the most likely reasons for control line
respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory failure. Review the procedure and repeat the test with a new test cassette. If
Store as packaged in the sealed pouch at the temperature (4-30℃ or
syndrome coronavirus (MERS-CoV) are zoonotic in origin and have been the problem persists, discontinue using the test kit immediately and contact
40-86℉). The kit is stable within the expiration date printed on the your local distributor.
linked to sometimes fatal illness.
labeling.
Coronaviruses are zoonotic, meaning they are transmitted between animals [QUALITY CONTROL]
Once open the pouch, the test should be used within one hour.
and people. Common signs of infection include respiratory symptoms,
Prolonged exposure to hot and humid environment will cause product A procedural control is included in the test. A colored line appearing in the
fever, cough, shortness of breath and breathing difficulties. In more severe
deterioration. control region (C) is considered an internal procedural control. It confirms
cases, infection can cause pneumonia, severe acute respiratory syndrome,
kidney failure and even death. The LOT and the expiration date were printed on the labeling. sufficient specimen volume, adequate membrane wicking and correct
Standard recommendations to prevent infection spread include regular [SPECIMEN] procedural technique.
hand washing, covering mouth and nose when coughing and sneezing, Control standards are not supplied with this kit. However, it is recommended
thoroughly cooking meat and eggs. Avoid close contact with anyone The test can be used to test Whole Blood/Serum/Plasma specimens. that positive and negative controls be tested as good laboratory practice to
showing symptoms of respiratory illness such as coughing and sneezing. To collect whole blood, serum or plasma specimens following regular confirm the test procedure and to verify proper test performance.
clinical laboratory procedures. [LIMITATIONS]
[PRINCIPLE]
Separate serum or plasma from blood as soon as possible to avoid
The MSLRDT100 IgG/IgM Rapid Test Cassette is a qualitative membrane hemolysis. Use only clear non-hemolyzed specimens. The MSLRDT100 IgG/IgM Rapid Test Cassette is limited to provide a
strip based immunoassay for the detection of antibodies (IgG and IgM) to Store specimens at 2-8℃ (36-46℉) if not tested immediately. Store qualitative detection. The intensity of the test line does not necessarily
Novel coronavirus in human Whole Blood/Serum/Plasma. The test cassette correlate to the concentration of the antibody in the blood.
specimens at 2-8℃ up to 7 days. The specimens should be frozen at
consists of: 1) a burgundy colored conjugate pad containing Novel The results obtained from this test are intended to be an aid in diagnosis
-20℃ (-4℉) for longer storage. Do not freeze whole blood specimens.
coronavirus recombinant envelope antigens conjugated with Colloid gold only. Each physician must interpret the results in conjunction with the
(Novel coronavirus conjugates), 2) a nitrocellulose membrane strip Avoid multiple freeze-thaw cycles. Prior to testing, bring frozen patient’s history, physical findings, and other diagnostic procedures.
containing two test lines (IgG and IgM lines) and a control line (C line). The specimens to room temperature slowly and mix gently. Specimens A negative test result indicates that antibodies to Novel coronavirus are
IgM line is pre-coated with the Mouse anti-Human IgM antibody, IgG line is containing visible particulate matter should be clarified by centrifugation either not present or at levels undetectable by the test.
coated with Mouse anti-Human IgG antibody. When an adequate volume of before testing.
Do not use samples demonstrating gross lipemia, gross hemolysis or
[PERFORMANCE CHARACTERISTICS]
test specimen is dispensed into the sample well of the test cassette, the
turbidity in order to avoid interference on result interpretation. Accuracy
specimen migrates by capillary action across the cassette. IgM anti-Novel
coronavirus, if present in the specimen, will bind to the Novel coronavirus [TEST PROCEDURE] A side-by-side comparison was conducted using the Novel coronavirus
conjugates. The immunocomplex is then captured by the reagent IgG/IgM Rapid Test and a leading commercial PCR. 120 clinical specimens
Allow the test device and specimens to equilibrate to temperature from Professional Point of Care site were evaluated. The following results
pre-coated on the IgM band, forming a burgundy colored IgM line, indicating
a Novel coronavirus IgM positive test result. IgG anti-Novel coronavirus if (15-30℃or 59-86℉) prior to testing. are tabulated from these clinical studies:
present in the specimen will bind to the Novel coronavirus conjugates. The 1. Remove the test cassette from the sealed pouch.
2. Hold the dropper vertically and transfer 1 drop of specimen to the MSLRDT100 IgG:
immunocomplex is then captured by the reagent coated on the IgG line,
forming a burgundy colored IgG line, indicating a Novel coronavirus IgG specimen well(S) of the test device, then add 2 drops of buffer PCR
MSLRDT100 IgG Total
positive test result. Absence of any T lines (IgG and IgM) suggests a (approximately 70μl) and start the timer. See the illustration below. Positive Negative
negative result. To serve as a procedural control, a colored line will always 3. Wait for colored lines to appear. Interpret the test results in 15 minutes. Positive 30 1 31
MSL
appear at the control line region indicating that proper volume of specimen Do not read results after 20 minutes. Negative 0 89 89
has been added and membrane wicking has occurred. Total 30 90 120
[WARNINGS AND PRECAUTIONS] A statistical comparison was made between the results yielding a sensitivity
1/2 109146201
of 100.00%, a specificity of 98.89% and an accuracy of 99.17%. each POL. The intra-assay agreements were 100%. The inter-site
agreement was 100 %.
MSLRDT100 IgM:
PCR
MSLRDT100 IgM Total
Positive Negative
Positive 27 2 29
MSL
Negative 3 88 91 30℃
Total 30 90 120 4℃
A statistical comparison was made between the results yielding a sensitivity
of 90.00%, a specificity of 97.78% and an accuracy of 95.83%.
Cross-Reactivity and Interference
1. Other common causative agents of infectious diseases were evaluated
for cross reactivity with the test. Some positive specimens of other
common infectious diseases were spiked into the Novel coronavirus
Guangzhou Medsinglong Medical Equipment Co., Ltd.
positive and negative specimens and tested separately. No cross
Add:Rm 405, South China Building, West Fuhua Road,
reactivity was observed with specimens from patients infected with HIV,
Shiqiao Town,Panyu,Guangzhou,511405 P.R China
HAV, HBsAg, HCV, HTLV, CMV, FLUA, FLUB, RSV and TP.
2. Potentially cross-reactive endogenous substances including common
serum components, such as lipids, hemoglobin, bilirubin, were spiked
at high concentrations into the Novel coronavirus positive and negative
specimens and tested, separately. No cross reactivity or interference
was observed to the device.
Specimens
Beautytec.nl Index of Symbol
Analytes Conc.
Positive Negative Do not reuse For in vitro diagnostic use only
Albumin 20mg/ml + - 30℃
Bilirubin 20μg/ml + - 4℃ Store between 4-30℃ Consult instructions for use
Hemoglobin 15mg/ml + -
Glucose 20mg/ml + - Caution Lot number
Uric Acid 200μg/ml + -
Lipids 20mg/ml + - Use by Contains sufficient for <n> tests
3. Some other common biological analytes were spiked into the Novel Keep away from sunlight Keep dry
coronavirus positive and negative specimens and tested separately. No
significant interference was observed at the levels listed in the table
Manufacturer Do not use if package is damaged
below.
Conc. Specimens
Analytes
(μg/ml) Positive Negative
Acetaminophen 200 + - Version No.: 1.0
Acetoacetic Acid 200 + - Effective Date: Feb. 28, 2020
Acetylsalicylic Acid 200 + -
Benzoylecgonine 100 + -
Caffeine 200 + -
EDTA 800 + -
Ethanol 1.0% + -
Gentisic Acid 200 + -
β - Hydroxybutyrate 20,000 + -
Methanol 10.0% + -
Phenothiazine 200 + -
Phenylpropanolamine 200 + -
Salicylic Acid 200 + -
Beautytec.nl
Reproducibility Harmoniestraat 8
Reproducibility studies were performed for Novel coronavirus IgG/IgM 4902PZ Oosterhout
Rapid Test at three physician office laboratories (POL). Sixty (60) clinical The Netherlands
serum specimens, 20 negative, 20 borderline positive and 20 positive, were TEL +31162853009
used in this study. Each specimen was run in triplicate for three days at info@beautytec.nl
2/2 109146201
You might also like
- Covid-19 Igg/Igm Rapid Test Cassette (WB/S/P) (Principle) : EnglishDocument2 pagesCovid-19 Igg/Igm Rapid Test Cassette (WB/S/P) (Principle) : Englishniluh suwasantiNo ratings yet
- Coronavirus CaseteDocument1 pageCoronavirus CaseteLidia NarbNo ratings yet
- Instructions For 2019-Ncov Ab Test (Colloidal Gold) : Product Name Intended UseDocument2 pagesInstructions For 2019-Ncov Ab Test (Colloidal Gold) : Product Name Intended UseDemograf27No ratings yet
- COVID-19 Rapid Test: Catalogue Number: RAPG-COV-019Document2 pagesCOVID-19 Rapid Test: Catalogue Number: RAPG-COV-019bernarNo ratings yet
- COVID-19 Rapid Test LeafletDocument2 pagesCOVID-19 Rapid Test LeafletAshraf PmNo ratings yet
- ICHROMA IgG-IgMDocument5 pagesICHROMA IgG-IgMAlfonso RamosNo ratings yet
- Biozek MachE PDFDocument15 pagesBiozek MachE PDFTjmita RunieNo ratings yet
- EUA Healgen Rapid Ifu PDFDocument4 pagesEUA Healgen Rapid Ifu PDFMuhammad Khairul HakimiNo ratings yet
- Tell Me Fast Novel Coronavirus (Covid-19) Igg/Igm Antibody TestDocument8 pagesTell Me Fast Novel Coronavirus (Covid-19) Igg/Igm Antibody TestDortmunderNo ratings yet
- Manual Dengue IgM Quanti CardDocument2 pagesManual Dengue IgM Quanti CardAjay NegiNo ratings yet
- For Professional and in Vitro Diagnostic Use OnlyDocument6 pagesFor Professional and in Vitro Diagnostic Use OnlySheilla DifaNo ratings yet
- D3. Manual Book EngDocument2 pagesD3. Manual Book EngMochamadSupriatnaNo ratings yet
- Manual Dengue Day 1 TestDocument2 pagesManual Dengue Day 1 TestCahiNo ratings yet
- Duo Dengue Ag-Igg/Igm Rapid Test: OnsiteDocument2 pagesDuo Dengue Ag-Igg/Igm Rapid Test: OnsiteCristian LaraNo ratings yet
- 08 IFU-Covid19 Neutralization AntibodyDocument2 pages08 IFU-Covid19 Neutralization AntibodyPhyo WaiNo ratings yet
- A6 COVID-19 Antigen-5002-EN-CE-2.0-单人份稀释液-109155102Document2 pagesA6 COVID-19 Antigen-5002-EN-CE-2.0-单人份稀释液-109155102HariNo ratings yet
- A Rapid Test For Detection of Dengue FeverDocument2 pagesA Rapid Test For Detection of Dengue FeverYvette TiongsonNo ratings yet
- Covid-19 Igg/Igm Rapid Test Cassette (Whole Blood/Serum/Plasma - Cassette) Product Code: Ccov-200Document2 pagesCovid-19 Igg/Igm Rapid Test Cassette (Whole Blood/Serum/Plasma - Cassette) Product Code: Ccov-200Thiago GalloNo ratings yet
- Covid-19 Igg/Igm Rapid Test Kit: Erick Esteban Paredes CedenoDocument2 pagesCovid-19 Igg/Igm Rapid Test Kit: Erick Esteban Paredes CedenoAlisonReinoso8No ratings yet
- OmanDocument133 pagesOmandprosenjitNo ratings yet
- SARS-CoV2 (COVID-19) IgG IgM C20200203Document2 pagesSARS-CoV2 (COVID-19) IgG IgM C20200203Mönica YauriNo ratings yet
- 2019-nCoV IgG-IgM RapiCardTM InstaTest (S-WB-P) InsertDocument3 pages2019-nCoV IgG-IgM RapiCardTM InstaTest (S-WB-P) InsertAlvaro Moina VelozNo ratings yet
- ™ Covid-19 Igg/Igm Rapid Test: OnsiteDocument2 pages™ Covid-19 Igg/Igm Rapid Test: OnsiteAlejandro ConaNo ratings yet
- Mono CassetteWSPDocument2 pagesMono CassetteWSPvictor sotoNo ratings yet
- COVID-19: Instructions For UseDocument2 pagesCOVID-19: Instructions For UseAKBAR SAPUTRANo ratings yet
- EUA Euroimmun ElisaG IfuDocument17 pagesEUA Euroimmun ElisaG IfuEdon BlakajNo ratings yet
- Article Andre AdiantoDocument7 pagesArticle Andre AdiantoAndre AdiantoNo ratings yet
- COVID-19 IgM-IgG WB Serum CassetteDocument5 pagesCOVID-19 IgM-IgG WB Serum CassetteIon CorbuNo ratings yet
- Performance Evaluation of A Dengue Iggigm and ns1 Rapid Test Device For Profesional in Vitro Diagnostic Use in Whole BloDocument3 pagesPerformance Evaluation of A Dengue Iggigm and ns1 Rapid Test Device For Profesional in Vitro Diagnostic Use in Whole BloHas SimNo ratings yet
- COVID-19: Instructions For UseDocument2 pagesCOVID-19: Instructions For UseTheresia IlyanNo ratings yet
- Innova SARS Cov 2 Antigen Test IFUDocument6 pagesInnova SARS Cov 2 Antigen Test IFUAnonymous yA5TGHNo ratings yet
- 2-Ifu-Test Kit PDFDocument3 pages2-Ifu-Test Kit PDFAlma AparicioNo ratings yet
- PACK INSERT-CoVCheck DIRECT-COVID-19 ANTIGEN Cassette Test-Ver-2Document4 pagesPACK INSERT-CoVCheck DIRECT-COVID-19 ANTIGEN Cassette Test-Ver-2Asif RasheedNo ratings yet
- 2-4.IFU-SARS-CoV-2 Saliva Antigen Rapid Test (Colloidal Gold Method)Document2 pages2-4.IFU-SARS-CoV-2 Saliva Antigen Rapid Test (Colloidal Gold Method)sunny niNo ratings yet
- 2 IFU 5513C Cellex QSARS CoV 2 IgGIgM Cassette Rapid TestDocument2 pages2 IFU 5513C Cellex QSARS CoV 2 IgGIgM Cassette Rapid TestdwiistantoNo ratings yet
- HIV 12 Stat Pak Dipstick Product Packet EnglishDocument6 pagesHIV 12 Stat Pak Dipstick Product Packet EnglishSagkyNo ratings yet
- D20rha25 2013-1Document2 pagesD20rha25 2013-1Icmi KadarsihNo ratings yet
- +AMS - 2019-Ncov IgG-IgM Device Brosure PDFDocument2 pages+AMS - 2019-Ncov IgG-IgM Device Brosure PDFsabinaantoniaNo ratings yet
- Laboratory Diagnosis For DengueDocument4 pagesLaboratory Diagnosis For DengueHamid RazaNo ratings yet
- Dengue IgG - IgmDocument4 pagesDengue IgG - Igmsatujuli23No ratings yet
- Anteceden Diagnostic Performance of Seven Rapid IgG IgM Antibody Tests and Theeuroimmun IgA IgG ELISA in COVID 19 PatientsDocument6 pagesAnteceden Diagnostic Performance of Seven Rapid IgG IgM Antibody Tests and Theeuroimmun IgA IgG ELISA in COVID 19 PatientsOmar Cucho GamboaNo ratings yet
- Cellex: Cellex Qsars-Cov-2 Igg/Igm Rapid TestDocument4 pagesCellex: Cellex Qsars-Cov-2 Igg/Igm Rapid TestIslamNo ratings yet
- Rp5331002 Incp-C81 Rightsign Ce en PiDocument1 pageRp5331002 Incp-C81 Rightsign Ce en PiHariNo ratings yet
- IFU Wondfo SARS CoV 2 Antibody Test (Lateral Flow Method) PDFDocument2 pagesIFU Wondfo SARS CoV 2 Antibody Test (Lateral Flow Method) PDFEndrio R HartonoNo ratings yet
- Use of Antibody Tests V6Document9 pagesUse of Antibody Tests V6Kenzo Adhi WiranataNo ratings yet
- Size: 137 X 218 MM: Rapid Test For The Detection of Dengue NS 1 Antigen in Human Serum/plasma DeviceDocument4 pagesSize: 137 X 218 MM: Rapid Test For The Detection of Dengue NS 1 Antigen in Human Serum/plasma DeviceMatibar RahmanNo ratings yet
- Rapid Test COVID-19 BSS Biosynex-Switzerland: For Professional in Vitro Diagnostic Use OnlyDocument6 pagesRapid Test COVID-19 BSS Biosynex-Switzerland: For Professional in Vitro Diagnostic Use Onlyno-replyNo ratings yet
- 3insert For COVID-19 IgM-IgG Antibody TestDocument7 pages3insert For COVID-19 IgM-IgG Antibody TestalexanderNo ratings yet
- Cellex Qsars-Cov-2 Iggigm PDFDocument2 pagesCellex Qsars-Cov-2 Iggigm PDFDiana TeranNo ratings yet
- Iso 13485 Iso 9001Document56 pagesIso 13485 Iso 9001Juliamm MartNo ratings yet
- Tuberculosis Igg/Igm Rapid Test Device (Serum/Plasma) : TH THDocument6 pagesTuberculosis Igg/Igm Rapid Test Device (Serum/Plasma) : TH THSheilla DifaNo ratings yet
- CTK Kit InsertDocument2 pagesCTK Kit Insertyousrazeidan1979No ratings yet
- Covid Fast Detection Kit Based On Colloidal GoldDocument2 pagesCovid Fast Detection Kit Based On Colloidal GoldJuan Carlos JumboNo ratings yet
- Cellex: Cellex Qsars-Cov-2 Igg/Igm Rapid TestDocument4 pagesCellex: Cellex Qsars-Cov-2 Igg/Igm Rapid Testapi-237132031No ratings yet
- Eurosurv 27 42 5Document13 pagesEurosurv 27 42 5Eben Leonel Albano MaiopueNo ratings yet
- Μπροσούρα rapid test GoldsiteDocument2 pagesΜπροσούρα rapid test GoldsiteMargaritaNo ratings yet
- Journal of Clinical Virology: SciencedirectDocument6 pagesJournal of Clinical Virology: SciencedirectVictor VargasNo ratings yet
- Covid-19: Testing TimesDocument2 pagesCovid-19: Testing TimeshgfksqdnhcuiNo ratings yet
- SARS-CoV-2 IgM&IgG Rapid Test PDFDocument2 pagesSARS-CoV-2 IgM&IgG Rapid Test PDFfatmaNo ratings yet
- Ncma215 - Nutrition and Diet Therapy: Week - 9Document4 pagesNcma215 - Nutrition and Diet Therapy: Week - 9ABEGAIL BALLORANNo ratings yet
- Evidence-Based Guideline Summary: Evaluation, Diagnosis, and Management of Facioscapulohumeral Muscular DystrophyDocument10 pagesEvidence-Based Guideline Summary: Evaluation, Diagnosis, and Management of Facioscapulohumeral Muscular DystrophyFitria ChandraNo ratings yet
- Worksheet 4, Ubaidillah, 3BDocument6 pagesWorksheet 4, Ubaidillah, 3BUbay SegaNo ratings yet
- Bilateral OsteoarthritisDocument58 pagesBilateral OsteoarthritisMaya VilNo ratings yet
- Congestion Pelvica 2020Document6 pagesCongestion Pelvica 2020Cristian RodríguezNo ratings yet
- Seminar: Pere Ginès, Aleksander Krag, Juan G Abraldes, Elsa Solà, Núria Fabrellas, Patrick S KamathDocument18 pagesSeminar: Pere Ginès, Aleksander Krag, Juan G Abraldes, Elsa Solà, Núria Fabrellas, Patrick S KamathcastillojessNo ratings yet
- The Apolinario Mabini Syphilis Rumors and Late 19th Century Philippine Power PlayDocument15 pagesThe Apolinario Mabini Syphilis Rumors and Late 19th Century Philippine Power PlayHaneul KImNo ratings yet
- KSPCB BIOMEDICAL POSTER 1aDocument1 pageKSPCB BIOMEDICAL POSTER 1armulmadgiNo ratings yet
- Chorea 9Document35 pagesChorea 9sanjay kamineniNo ratings yet
- Clinical Features and Diagnosis of Abdominal Aortic Aneurysm - UpToDateDocument53 pagesClinical Features and Diagnosis of Abdominal Aortic Aneurysm - UpToDateALVARO ARIASNo ratings yet
- EASL-GL Benign Tumor LiverDocument13 pagesEASL-GL Benign Tumor LiveroliviaNo ratings yet
- UntitledDocument17 pagesUntitledInggrid Aprilia ChristyNo ratings yet
- Detoxification:Management of AmaDocument3 pagesDetoxification:Management of AmaqueencelNo ratings yet
- Gastroenterology Evaluation TemplateDocument2 pagesGastroenterology Evaluation Templatee-MedTools86% (7)
- New Health Care Clinical: Laboratory SrinagarDocument1 pageNew Health Care Clinical: Laboratory SrinagarRajaNo ratings yet
- An Approach To A Floppy InfantDocument33 pagesAn Approach To A Floppy InfantayunisallehNo ratings yet
- TM Joint PDFDocument16 pagesTM Joint PDFdhruvNo ratings yet
- Case ReportDocument11 pagesCase ReportBerhanu DigamoNo ratings yet
- r145274208 Isabella Fonseca CUR145274208Document1 pager145274208 Isabella Fonseca CUR145274208Isabella FonsecaNo ratings yet
- Counseling TechniquesDocument60 pagesCounseling Techniquesdinalen0% (1)
- Lesson Plan On Spina BifidaDocument24 pagesLesson Plan On Spina BifidaPriyaNo ratings yet
- Major Depressive Disorder Symptoms and Prevalence For IB Abnormal PsychologyDocument13 pagesMajor Depressive Disorder Symptoms and Prevalence For IB Abnormal PsychologychrissybissyNo ratings yet
- Axa Group Corporate PresentationDocument35 pagesAxa Group Corporate PresentationRajneesh VermaNo ratings yet
- 1.0 Thrombocytes SCDocument10 pages1.0 Thrombocytes SC西矢椛No ratings yet
- Minor AilmentsDocument14 pagesMinor AilmentsGazala100% (1)
- Closed Fracture of Left Femoral Neck: Case ReportDocument31 pagesClosed Fracture of Left Femoral Neck: Case Reporttari nurulNo ratings yet
- Concept Map PTBDocument1 pageConcept Map PTBJoan Abardo100% (2)
- PRE Test 1Document15 pagesPRE Test 1Naomi VirtudazoNo ratings yet
- Jamaophthalmology Biswas 2016 Oi 160055Document8 pagesJamaophthalmology Biswas 2016 Oi 160055Ghalia HayetNo ratings yet
- Obstetrics Midterms Rationale 2nd Sem 2018 2019Document21 pagesObstetrics Midterms Rationale 2nd Sem 2018 2019Gene Paulo UyNo ratings yet