Professional Documents
Culture Documents
PIIS2213260020302435
PIIS2213260020302435
Uploaded by
Amgel FloresCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PIIS2213260020302435
PIIS2213260020302435
Uploaded by
Amgel FloresCopyright:
Available Formats
Articles
Pulmonary and cardiac pathology in African American
patients with COVID-19: an autopsy series from New Orleans
Sharon E Fox, Aibek Akmatbekov, Jack L Harbert, Guang Li, J Quincy Brown, Richard S Vander Heide
Summary
Background Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread rapidly across the USA, causing Lancet Respir Med 2020
extensive morbidity and mortality, particularly in the African American community. Autopsy can considerably Published Online
contribute to our understanding of many disease processes and could provide crucial information to guide May 27, 2020
https://doi.org/10.1016/
management of patients with coronavirus disease 2019 (COVID-19). We report on the relevant cardiopulmonary S2213-2600(20)30243-5
findings in, to our knowledge, the first autopsy series of ten African American decedents, with the cause of death
See Online/Comment
attributed to COVID-19. https://doi.org/10.1016/
S2213-2600(20)30244-7
Methods Autopsies were performed on ten African American decedents aged 44–78 years with cause of death Department of Pathology,
attributed to COVID-19, reflective of the dominant demographic of deaths following COVID-19 diagnosis in New Louisiana State University
Health Sciences Center,
Orleans. Autopsies were done with consent of the decedents’ next of kin. Pulmonary and cardiac features were
New Orleans, LA, USA
examined, with relevant immunostains to characterise the inflammatory response, and RNA labelling and electron (S E Fox MD, A Akmatbekov MD,
microscopy on representative sections. J L Harbert MD,
Prof R S Vander Heide MD);
Pathology and Laboratory
Findings Important findings include the presence of thrombosis and microangiopathy in the small vessels and
Medicine Service, Southeast
capillaries of the lungs, with associated haemorrhage, that significantly contributed to death. Features of diffuse Louisiana Veterans Healthcare
alveolar damage, including hyaline membranes, were present, even in patients who had not been ventilated. Cardiac System, New Orleans, LA, USA
findings included individual cell necrosis without lymphocytic myocarditis. There was no evidence of secondary (S E Fox); Department of
Biomedical Engineering, Tulane
pulmonary infection by microorganisms.
University, New Orleans, LA,
USA (G li MS,
Interpretation We identify key pathological states, including thrombotic and microangiopathic pathology in the lungs, J Quincy Brown PhD)
that contributed to death in patients with severe COVID-19 and decompensation in this demographic. Management Correspondence to:
of these patients should include treatment to target these pathological mechanisms. Dr Sharon E Fox and
Prof Richard S Vader Heide,
Department of Pathology,
Funding None. Louisiana State University Health
Sciences Center, New Orleans,
Copyright © 2020 Elsevier Ltd. All rights reserved. LA 70112, USA
sfox3@lsuhsc.edu;
rvand3@lsuhsc.edu
Introduction implications for treatment of severe disease in this
The first confirmed case of Severe acute respiratory patient population.
syndrome coronavirus 2 (SARS-CoV-2) infection in
the USA was reported on Jan 20, 2020. Since then, Brief clinical summary
the virus has spread across the USA, and several Patients were men and women aged 44–78 years. All were
cities have become epicentres of the pandemic. As of identified as African American by family or self-
March 31, 2020, the Louisiana Department of Health identification in the hospital demographic record. All
reported a total of 5237 coronavirus disease 2019 patients had at least one comorbidity, the most common For more on LA state records see
(COVID-19) cases, with 1355 hospital admissions and of which were hypertension, controlled by medication, https://ldh.la.gov/
239 COVID-19-related deaths statewide, and 1834 cases type 2 diabetes, and obesity (for full patient characteristics,
and 101 deaths in the city of New Orleans—among the see appendix pp 2–4). Only one patient was known to be See Online for appendix
highest rates of death by population in the USA. African immunosuppressed.
Americans represent more than 70% of deaths related In all patients, the clinical course consisted of
to COVID-19 in Louisiana, a trend that could have both approximately 3–7 days of mild cough and fever of
socioeconomic and biological causes. The University 38·3–38·8°C, with sudden respiratory decompensation
Medical Center in New Orleans, built after Hurricane just before arrival to the emergency department, or
Katrina, is equipped with an autopsy suite that meets sudden collapse at home (appendix pp 2–4). Chest x-rays,
US Centers for Disease Control standards for autopsy where available, revealed bilateral ground-glass opacities,
of patients positive for COVID-19. We report here on consistent with acute respiratory distress syndrome
the cardiopulmonary findings of the first autopsy (ARDS), that worsened over the hospital course. Patients
series, to our knowledge, of ten African Americans with reporting to the emergency department were intubated
recorded cause of death of COVID-19. The distinctive and admitted to the intensive care unit (ICU). One patient
pathological findings are likely to have important (appendix, patient 6) on presentation to the emergency
www.thelancet.com/respiratory Published online May 27, 2020 https://doi.org/10.1016/S2213-2600(20)30243-5 1
Articles
Research in context
Evidence before this study Added value of this study
We reviewed the single study of autopsy in a COVID-19 positive This study presents a large series of autopsies of whole organs,
patient by Z Xu and colleagues, published in this journal, and within a specific demographic with a high rate of adverse
reports of pathology from severe acute respiratory syndrome outcomes within the USA.
(SARS) coronavirus and similar viral infections by J Nicholls.
Implications of all the available evidence
We reviewed the publicly available literature on coronavirus
The key implications include a mechanism for severe pathology
disease 2019 (COVID-19) pathology, including reports from
within the African American population, probably extendable
China and Europe. We searched PubMed and Google Scholar for
to all patients with severe disease, and possibly a target for
literature published between March 24, and April 8, 2020.
immediate therapeutic management. The results might also be
Search terms used included COVID-19, SARS-CoV-2,
applicable to a broader demographic with severe COVID-19.
coronavirus, and pathology or autopsy.
despite support, the families chose to withdraw care.
A B Consent for autopsy without restriction was given by the
next of kin, and the studies within this report were
determined to be exempt from oversight by the
Institutional Review Board at Louisiana State University
Health Sciences Center and Tulane University.
Role of the funding source
There was no funding source for this study. The
corresponding author had full access to all data and the
C
final responsibility to submit for publication.
Results
Gross findings
Gross examination of the lungs at the time of autopsy
Figure 1: Gross findings of the lungs and heart
revealed the tracheae to be of normal calibre and mildly
(A) Lungs with bilateral pulmonary oedema and patches of dark haemorrhage. (B) Cut sections of lung showing erythematous. In all but one patient, the lungs were more
thrombi present within peripheral small vessels (green arrows) (C) A heart showing extreme right ventricular than 1 SD heavier than normal weight (appendix p 6).
dilatation, with straightening of the interventricular septum. The pulmonary arteries at the hilum of each lung were
free of thromboemboli. The bronchi revealed thick, white
department had hypoxic respiratory failure and developed mucus in the lungs of one patient, and pink froth in the
ventricular tachycardia, leading to death before receiving airways of the others. Mild to moderate serosanguinous
ventilator support. All the patients tested positive for pericardial and pleural effusions were also present. The
SARS-CoV-2 (by SARS-CoV-2 RT-PCR). Treatment in the parenchyma of each lung was diffusely oedematous and
ICU initially included vancomycin, azithromycin, and firm, con sistent with the clinical diagnosis of ARDS.
cefepime for all patients, with one patient receiving Regions of dark-coloured haemorrhage with focal
dexamethasone, and two patients later receiving hydroxy demarcation were identified throughout the peripheral
chloroquine (appendix pp 2–4). Notable laboratory parenchyma in the lungs of all but one of the decedents
findings were the frequent develop ment of elevated (figure 1A). On cut sections, the areas identified as
ferritin and fibrinogen, but with minimal prolongation haemorrhagic on the external surface showed frank
of prothrombin time, and no elevation of partial haemorrhage. After fixation, the cut surfaces of the lung
thromboplastin time (appendix p 5). Within 24 h of death, tissue showed alternating areas of tan-grey consolidation
an increased neutrophil count with relative lymphopenia with patchy areas of haemorrhage 3–6 cm in maximal
was observed in some patients (appendix p 5). Glucose diameter. In all patients, small, firm thrombi were
and creatinine concentrations increased above baseline in present in sections of the peripheral parenchyma
all but two patients, and aspartate aminotransferase (figure 1B). Only in a patient on immunosuppression
concentrations became slightly elevated in four patients. was there focal consolidation—the remainder of the
D-dimer concentrations taken near the time of death were lungs showed no evidence of lobar infiltrate, abscess, or
markedly elevated in all patients when this was measured definitive gross inflammatory process.
(n=6, range 249–47 559 ng/mL; appendix p 5). Detailed Examination of the heart was done in nine autopsies
laboratory findings from all patients are included in the (appendix p 6). The decedents’ hearts weighed
appendix (p 5). When the patients continued to deteriorate 370–600 g. The cut surfaces of the myocardium were
2 www.thelancet.com/respiratory Published online May 27, 2020 https://doi.org/10.1016/S2213-2600(20)30243-5
Articles
firm, red-brown, and free of significant lesions in all
A B
patients, and the coronary arteries showed no significant
stenosis or acute thrombus formation. The most
significant gross findings were cardiomegaly and right
ventricular dilatation. In several patients, massive
dilatation could be seen; for example, in one decedent,
the right ventricular cavity was 3·6 cm in diameter and
the left ventricle was 3·4 cm at its greatest diameter
(figure 1C). Dilatation of the right ventricle was often 200 µm 100 µm
associated with elevated brain natriuretic peptide (BNP),
although in the example in figure 1B, troponin con C D
centration increased 3 days before death with a relative
decline over the next 9 days, and BNP was not repeated
(appendix p 5, patient 2).
Microscopic findings
The lungs were extensively sampled across central and
peripheral regions of each lobe bilaterally. Samples from
200 µm 80 µm
each patient were immunostained. Histological exam
ination of the lungs showed bilateral diffuse alveolar
Figure 2: Pulmonary diffuse alveolar damage
damage in all patients (figure 2). Alveolar damage was in All patients had extensive diffuse alveolar damage. (A) Green arrows indicate early hyaline membranes in a patient
the early exudative phase with oedema and early hyaline with 1 week of symptomatic illness and no mechanical ventilation (H&E stain). (B) Green arrows indicate extensive
membrane formation in two patients (figure 2A), in the hyaline membranes and fibrinous exudate in a patient with 9 days of symptomatic illness, including 6 days of
ventilation (H&E stain). (C) Green arrow indicates dense hyaline membranes, with organising fibrosis (green
transition to proliferative phases in seven patients
arrowhead), and fibrin thrombi present in small vessels (blue arrows), with a pauci-immune and oedematous
(figure 2B), and in clear proliferative to fibrotic phase in background in a patient after 32 days of illness, including 25 days on ventilatory support. Extensive haemorrhage was
one patient (figure 2C). One patient died before receiving also present (H&E stain). (D) Bronchial respiratory epithelium shown with cilia present, and absence of squamous
ventilator support, and one patient received only 1 day metaplasia in a patient receiving ventilatory support for 6 days. H&E=haematoxylin and eosin.
of ventilator support (appendix p 3, patients 6 and 7).
These two patients had markedly elevated D-dimers
A
(appendix p 5), with evidence of pulmonary micro
thrombi and early hyaline membrane formation
(figure 2A). The bronchial respiratory mucosa was largely
intact, with microvilli present and no evidence of
squamous metaplasia (figure 2D), which differs from the
pathology seen in the first SARS epidemic.1 The
inflammatory cell infiltrate was composed of a mixture of 900 µm 600 µm
CD4+ and CD8+ lymphocytes (figure 3D and appendix, p 7),
located predominantly in the interstitial spaces and
around larger bronchioles and blood vessels. CD4+ B C D
lymphocytes could be seen in aggregates around small
vessels, some of which appeared to contain platelets and
small thrombi (figure 3). All but one patient had foci of
haemorrhage. Desquamated type 2-pneumocytes with
apparent viral cytopathic effect consisting of cytomegaly 60 µm
and enlarged nuclei, with bright, eosinophilic nucleoli,
were present within alveolar spaces (figure 4). The largest Figure 3: Pulmonary thrombi and microangiopathy
(A) Thrombus in a small pulmonary artery (green arrow), with small thrombus seen in adjacent pulmonary venule
of these cells in most patients contained a granular (green arrowhead), with H&E present on the left, and CD61 immunostain highlighting platelets within the thrombi
cytoplasm, probably representing viral effect similar to on the right. (B) Many megakaryocytes were present within the small vessels and alveolar capillaries (green arrow).
that reported in the first SARS epidemic.1 Scattered (C) CD61 immunostain highlighting additional fibrin and platelet thrombus shown in a small vessel, with
hyaline membranes could be seen, as well as fibrin megakaryocyte stained below (green arrowhead). Von Willebrand Factor immunostain additionally highlighted
these vessels (appendix p 8). (D) Small, perivascular aggregates of lymphocytes. Also present were small
deposition, consistent with fibrinous diffuse alveolar lymphocytic aggregates surrounding airways, which were positive for CD4 immunostain, with only scattered CD8
damage. The alveolar capillaries were notably thickened, positive cells present (appendix p 7).
with surrounding oedema, and fibrin thrombi were
present within the capillaries and small vessels (figure 3). nuclear hyperchromasia and atypia. These cells were
A notable finding was the presence of CD61+ mega located within alveolar capillaries, and could be seen
karyocytes (figure 3B & 3C], possibly representing actively producing platelets. The fibrin and platelets
resident pulmonary megakaryocytes, with significant present within small vessels also appeared to aggregate
www.thelancet.com/respiratory Published online May 27, 2020 https://doi.org/10.1016/S2213-2600(20)30243-5 3
Articles
A B E
C D
0·2 µm
Figure 4: SARS-CoV-2 cytopathic effects
(A) Several enlarged pneumocytes within a damaged alveolus, having enlarged nuclei, prominent nucleoli, and cytologic atypia (H&E stain). (B) Relative distribution
of DNA (red) versus RNA (green) in tissue sections via DRAQ5 and SYTO RNASelect fluorescent staining (appendix p 1 for staining details). Pneumocytes with
increased RNA in alveolar spaces show aggregation; enlarged, atypical morphology shown by DNA stain; and abundant RNA present within the cytoplasm (green
arrows). (C) Entrapment of immune cells, including degenerated neutrophils, within fibrin, and strands of extracellular material with weak DNA staining. (D) Control
lung tissue obtained at autopsy for non-pulmonary cause of death before the coronavirus disease 2019 pandemic. (E) Electron microscopy of the lung, showing
particles suggestive of viral infection (examples highlighted by blue arrows. H&E=haematoxylin and eosin.
inflammatory cells, with entrapment of many neutro
phils. A patient on immunosuppression, had evidence of
a focal acute inflammatory infiltrate suggestive of a
A secondary infection. The neutrophils in this patient’s
i ii sample, however, were partly degenerated and entrapped
iii
in fibres, possibly representing neutrophil extracellular
traps (figure 4C),2,3 and were seen together with clusters
of CD4+ mononuclear cells. No significant neutrophilic
infiltrate was identified within airways or the interstitium
to suggest secondary infection in the other patients.
100 µm The sections of myocardium did not show any large or
confluent areas of myocyte necrosis (figure 5). Cardiac
B histopathology was remarkable, however, for scattered
individual cell myocyte necrosis in each heart exam
i ii iii ined. In rare areas, lymphocytes were adjacent to, but
not surrounding, degenerating myocytes. Whether this
observation represents an early manifestation of viral
myocarditis is not certain, but there was no significant
brisk lymphocytic inflammatory infiltrate suggestive of
200 µm viral myocarditis.These observations could be consistent
with a paper by Chen and colleagues,4 in which the
authors hypothesise that pericytes might be infected by
Figure 5: Cardiac microscopic findings
A) patient 2, i: Cardiac myocytes, ii: CD4 immunostain, iii: CD8 immunostain. B) patient 3, i: Cardiac myocytes, ii: CD31 SARS-CoV-2 and cause capillary endothelial cell or
immunostain, iii: CD4 immunostain. Cardiac myocytes showing focal, atypical myocyte degeneration (green arrows), microvascular dysfunction that could cause individual
H&E stain (sample images from patients aged 44 and 63 years receiving azithromycin but not hydroxychloroquine). cell necrosis. There was no obvious viral cytopathic
Scant lymphocytes were present within the interstitial and endothelial spaces, with slightly more CD4+ than CD8+ cells
on visual inspection of immunostains. A CD31 immunostain highlighted endothelial cells, with focal prominence (green
effect by light microscopy, but direct viral infection
arrow) that appeared non-specific. CD4+ lymphocytes were occasionally seen in a non-specific pattern within the of myocytes cannot be ruled out in this limited
coronary artery intima. H&E=haematoxylin and eosin. examination.
4 www.thelancet.com/respiratory Published online May 27, 2020 https://doi.org/10.1016/S2213-2600(20)30243-5
Articles
Discussion ventricular dilatation. The underlying cause of scattered
The dominant pathological process in all lungs exam atypical myocyte degeneration remains uncertain.
ined was diffuse alveolar damage, accompanied by Previous evidence10–14 reports viral infection causing
thrombosed small vessels with significant associated activation of both maladaptive cytokine pathways and a
haemorrhage. An important additional mechanism that platelet response, and our findings suggest that these
contributed to death in this initial series of autopsies was immune functions might be related to severe forms of
a thrombotic microangiopathy involving the lungs. COVID-19. In response to systemic and pulmonary viral
Thrombi were not grossly apparent in any other organs infections of H1N1 influenza and dengue, megakaryocytes
examined, including kidney, spleen, pancreas, and liver. have been known to respond by overexpressing IFITM3
This thrombotic process might involve activation of and producing platelets with the same overexpression.10
megakaryocytes, possibly those native to the lung, with In addition, platelets and megakaryocytes might have
platelet aggregation and platelet-rich clot formation, in receptors for viruses,11–14 some of which can be specifically
addition to fibrin deposition. Small vessel thrombus activated in H1N1 influenza, often in association with
formation in the lung periphery was often associated lymphopenia.15–17 Some evidence18 suggests that the
with foci of alveolar haemorrhage. This finding is likely previous SARS-CoV directly infected megakaryocytes,
to be associated with the fact that when measured, and that platelet function was affected in damaged lungs
D-dimer was elevated in all but one patient, although of those with SARS. We do not have evidence of direct
there was minimal prolongation of prothrombin time infection of megakaryocytes by SARS-CoV-2, but the
and no prolongation of partial thromboplastin time, with abundance of these cells in the lungs at autopsy is
only one patient showing thrombocytopenia, suggesting probably related to the abundance of small, sometimes
a coagulative pathology other than disseminated intra platelet-rich thrombi, and foci of haemorrhage.
vascular coagulation. Several patients who had a more A notable finding was the absence of observed
protracted hospital course had extensive fibrin and early secondary infection in our patients. Although most of the
organisation, with degenerated neutrophils within the patients received antibiotic therapy throughout their
alveoli possibly representing neutrophil extracellular hospital courses, the absence of bacterial or fungal
traps.2,3 Increased macrophage presence within alveoli infection suggests that this was not the main cause of
could also be seen with advancement of disease death. In addition, despite the presence of lymphopenia
(appendix p 7). The findings of diffuse alveolar damage in most patients, we observed a mild lymphocytic
and enlarged, atypical pneumocytes are consistent with infiltrate in the lungs, composed primarily of CD4+ cells,
autopsy reports from the first SARS epidemic,1 but the so lymphocyte sequestration in the lungs was probably
burden of small vessel pulmonary thrombi and platelet not a major cause of lymphopenia in our cohort. We also
aggregation is a novel and important finding specific to note that two of our patients were aged 40–50 years,
SARS-CoV-2, and extends beyond what is typically seen younger than those generally thought to be at risk of
in diffuse alveolar damage. These findings could suggest death due to COVID-19. These two patients had no
endothelial damage or platelet dysfunction within the history of immunosuppressive therapy, although they
pulmonary vasculature, as seen in reports of endotheliitis did have obesity, hypertension, and diabetes—co
caused by SARS-CoV-2.5 On RNA imaging, we were able morbidities often present in our patient population in
to visualise fused pneumocytes within alveolar spaces, New Orleans and in the population of other cities with
which contained abundant RNA and probably repres large and rising burdens of COVID-19. We believe that
ented virally infected cells. effective therapy for this patient demographic—and
Electron microscopy of lung tissue revealed changes probably patients with severe infection across demo
likely due to viral infection (figure 4). These may graphics—should target not only the viral pathogen, but
represent the cells previously described from a report of also the thrombotic and microangiopathic effects of the
post mortem biopsy from a decedent in China.6 virus, and possibly a maladaptive immune response to
Cardiac findings were notable for absence of lympho viral infection.
cytic myocarditis. Many early reports have indicated that Contributors
fulminant myocarditis occurs in SARS-CoV-2 infection7–9 SEF contributed to performance of autopsies, including tissue selection,
and contributes significantly to the morbidity of interpretation of histology slides, literature search, histology figures,
data analysis, data interpretation, and first draft of the manuscript. GL
COVID-19. Based on these reports and the troponin data contributed to data collection, analysis and interpretation of fluorescent
from our decedents that suggest little myocardial stains, creation of fluorescent image figure, and methods in the
damage (appendix p 5), we were interested in find out manuscript. AA and JLH contributed to the performance of autopsies,
whether hearts would show significant lymphocytic selected tissue for histology and staining, and contributed to tables,
figures, data collection, and editing the manuscript. JQB contributed to
infiltrates. We saw neither lymphocytic infiltrates, nor data collection, data analysis, data interpretation, and editing the
large areas of myocyte necrosis. Although SARS-CoV-2 manuscript. RSVH contributed to the performance of autopsies,
infection did not cause myocarditis in this cohort, sampling and analysis of tissue, interpretation and analysis of heart and
further studies are needed. The rise in BNP observed in lung sections, electron microscopic data analysis and figures, and
contributed to writing and editing of the manuscript.
several of our patients was probably due to acute right
www.thelancet.com/respiratory Published online May 27, 2020 https://doi.org/10.1016/S2213-2600(20)30243-5 5
Articles
Declaration of interests 9 Wei X, Fang Y, Hu H. Immune-mediated mechanism in
We declare no competing interests. coronavirus fulminant myocarditis. Eur Heart J 2020; published
online April 22. DOI:10.1093/eurheartj/ehaa333.
Acknowledgments 10 Campbell RA, Schwertz H, Hottz ED, et al. Human megakaryocytes
We thank our patients and their families, who in a time of loss have possess intrinsic antiviral immunity through regulated induction of
sought to help others understand this disease. We are also grateful for IFITM3. Blood 2019; 133: 2013–26.
the support of the Department of Pathology at Louisiana State University 11 Youssefian T, Drouin A, Massé J-M, Guichard J, Cramer EM.
Health Sciences Center, and for the hard work of the University Medical Host defense role of platelets: engulfment of HIV and
Center staff; in particular, Nicole Bichsel, for her invaluable role as Staphylococcus aureus occurs in a specific subcellular compartment
autopsy assistant. Finally, we would like to acknowledge and is enhanced by platelet activation. Blood 2002; 99: 4021–29.
Dr Paula L Bockenstedt, Associate Professor of Hematology at the 12 Boukour S, Massé J-M, Bénit L, Dubart-Kupperschmitt A,
University of Michigan, for her expertise and guidance in this work. Cramer EM. Lentivirus degradation and DC-SIGN expression by
human platelets and megakaryocytes. J Thromb Haemost 2006;
References 4: 426–35.
1 Nicholls JM, Poon LLM, Lee KC, et al. Lung pathology of fatal
severe acute respiratory syndrome. Lancet 2003; 361: 1773–78. 13 Loria GD, Romagnoli PA, Moseley NB, Rucavado A, Altman JD.
Platelets support a protective immune response to LCMV by
2 Mikacenic C, Moore R, Dmyterko V, et al. Neutrophil extracellular preventing splenic necrosis. Blood 2013; 121: 940–50.
traps (NETs) are increased in the alveolar spaces of patients with
ventilator-associated pneumonia. Crit Care 2018; 22: 358. 14 Middleton EA, Weyrich AS, Zimmerman GA. Platelets in
pulmonary immune responses and inflammatory lung diseases.
3 Lefrançais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Physiol Rev 2016; 96: 1211–59.
Maladaptive role of neutrophil extracellular traps in pathogen-
induced lung injury. JCI Insight 2018; 3: e98178. 15 Rondina MT, Brewster B, Grissom CK, et al. In vivo platelet
activation in critically ill patients with primary 2009 influenza
4 Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in A(H1N1). Chest 2012; 141: 1490–95.
human heart indicates new potential mechanism of heart injury
among patients infected with SARS-CoV-2. Cardiovasc Res 2020; 16 Khandaker G, Dierig A, Rashid H, King C, Heron L, Booy R.
published online March 30. DOI:10.1093/cvr/cvaa078. Systematic review of clinical and epidemiological features of the
pandemic influenza A (H1N1) 2009. Influenza Other Respi Viruses
5 Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and 2011; 5: 148–56.
endotheliitis in COVID-19. Lancet 2020; 395: 1417–18.
17 Gomez-Casado C, Villaseñor A, Rodriguez-Nogales A, Bueno JL,
6 Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 Barber D, Escribese MM. Understanding platelets in infectious and
associated with acute respiratory distress syndrome. allergic lung diseases. Int J Mol Sci 2019; published online April 8.
Lancet Respir Med 2020; 8: 420–22. DOI:10.3390/ijms20071730.
7 Kim I-C, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in 18 Yang M, Ng MHL, Li CK. Thrombocytopenia in patients with severe
a 21-year-old female patient. Eur Heart J 2020; published online acute respiratory syndrome (review). Hematology 2005; 10: 101–05.
April 13. DOI:10.1093/eurheartj/ehaa288.
8 Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis
saved with glucocorticoid and human immunoglobulin. Eur Heart J
2020; published online March 16. DOI:10.1093/eurheartj/ehaa190.
6 www.thelancet.com/respiratory Published online May 27, 2020 https://doi.org/10.1016/S2213-2600(20)30243-5
You might also like
- Post-Viral Effects of COVID-19 in The Olfactory System and Their ImplicationsDocument9 pagesPost-Viral Effects of COVID-19 in The Olfactory System and Their ImplicationsJorge Augusto Israel 狼No ratings yet
- An Investigation Into The WIV Databases That Were Taken of IneDocument26 pagesAn Investigation Into The WIV Databases That Were Taken of InelaotanNo ratings yet
- English 9 Activity Sheet: Quarter 3 - MELC 3Document9 pagesEnglish 9 Activity Sheet: Quarter 3 - MELC 3rai genolos67% (9)
- Contingency Plan: Madapdap Resettlement High SchoolDocument12 pagesContingency Plan: Madapdap Resettlement High SchoolHacky Tot100% (2)
- Mckinsey Report - COVID-19-Facts-and-Insights-March-25 PDFDocument57 pagesMckinsey Report - COVID-19-Facts-and-Insights-March-25 PDFUmang K100% (2)
- COVID-19 LEGACY: SARS-CoV-2 clinical trials, vaccines trials and bioethicsFrom EverandCOVID-19 LEGACY: SARS-CoV-2 clinical trials, vaccines trials and bioethicsNo ratings yet
- 1 s2.0 S2213260020302435 MainDocument6 pages1 s2.0 S2213260020302435 MainranauliafkNo ratings yet
- Journal Pre-Proof: JACC: Basic To Translational ScienceDocument6 pagesJournal Pre-Proof: JACC: Basic To Translational ScienceAjideniNo ratings yet
- Journal Pre-Proof: Best Practice & Research Clinical AnaesthesiologyDocument62 pagesJournal Pre-Proof: Best Practice & Research Clinical AnaesthesiologyMohammed Shuaib AhmedNo ratings yet
- Outcomes and Risk Factors For Cardiovascular Events in Hospitalized COVID-19 PatientsDocument13 pagesOutcomes and Risk Factors For Cardiovascular Events in Hospitalized COVID-19 Patientslaura_d_2No ratings yet
- PIIS0140673622003270Document7 pagesPIIS0140673622003270chaivat kNo ratings yet
- SARS-CoV-2 Viremia Is Associated With Distinct Proteomic Pathways and Predicts COVID-19 OutcomesDocument13 pagesSARS-CoV-2 Viremia Is Associated With Distinct Proteomic Pathways and Predicts COVID-19 OutcomesYoga Nugraha WNo ratings yet
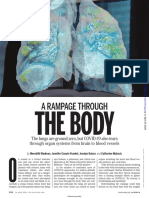
- A Rampage Through: The BodyDocument5 pagesA Rampage Through: The BodyEdmundo CadilhaNo ratings yet
- 2021 - Ochoa Et Al - HCWDocument43 pages2021 - Ochoa Et Al - HCWMario Martínez TorijaNo ratings yet
- Covid-19 in Critically Ill Patients in The Seattle Region - Case SeriesDocument18 pagesCovid-19 in Critically Ill Patients in The Seattle Region - Case SeriesZelgi PutraNo ratings yet
- Journal of Critical CareDocument5 pagesJournal of Critical CareUnomoshNo ratings yet
- Jama Sanders 2020 RV 200005 PDFDocument13 pagesJama Sanders 2020 RV 200005 PDFPuty Annisa PrilinaNo ratings yet
- Jama Sanders 2020 RV 200005Document13 pagesJama Sanders 2020 RV 200005nanaNo ratings yet
- Sepsis and Septic Shock: June 2016Document22 pagesSepsis and Septic Shock: June 2016FriskaNo ratings yet
- Jurnal Covid 19Document46 pagesJurnal Covid 19Adeta Yuniza100% (1)
- Ni Hms 794087Document24 pagesNi Hms 794087Croitoru CosminNo ratings yet
- Critical Care For Multiple Organ Failure SecondaryDocument20 pagesCritical Care For Multiple Organ Failure SecondarysazaperdanaNo ratings yet
- COVID-19-Induced Neurovascular Injury: A Case Series With Emphasis On Pathophysiological MechanismsDocument17 pagesCOVID-19-Induced Neurovascular Injury: A Case Series With Emphasis On Pathophysiological MechanismsAji Prasetyo UtomoNo ratings yet
- Artigo Pra Ler 2Document27 pagesArtigo Pra Ler 2Luiz ClaudioNo ratings yet
- 2.prevalence and Impact of - Myocardial InjuryDocument14 pages2.prevalence and Impact of - Myocardial Injuryrest_a2003No ratings yet
- MainDocument9 pagesMainDeny NoviantoroNo ratings yet
- Price Et Al, Hospitalization and Mortality Among Black Patients and White Patients With Covid-19Document10 pagesPrice Et Al, Hospitalization and Mortality Among Black Patients and White Patients With Covid-19Constanzza Arellano LeivaNo ratings yet
- 20220623135722-Artigo - Covid 19 e OutrasoençasDocument43 pages20220623135722-Artigo - Covid 19 e Outrasoençasmarcos crescencioNo ratings yet
- Sphingomonas KoreensisDocument11 pagesSphingomonas KoreensisSMIBA MedicinaNo ratings yet
- Definition of SIRS, Sepsis, Severe SepsisDocument9 pagesDefinition of SIRS, Sepsis, Severe SepsisSara NicholsNo ratings yet
- New Insights of Emerging Sars-Cov-2: Epidemiology, Etiology, Clinical Features, Clinical Treatment, and PreventionDocument22 pagesNew Insights of Emerging Sars-Cov-2: Epidemiology, Etiology, Clinical Features, Clinical Treatment, and PreventionWendi IochNo ratings yet
- Predictors of COVID-19 Severity: A Literature ReviewDocument10 pagesPredictors of COVID-19 Severity: A Literature ReviewRafiqua Sifat JahanNo ratings yet
- Journal Pre-Proof: Diagnostic Microbiology & Infectious DiseaseDocument97 pagesJournal Pre-Proof: Diagnostic Microbiology & Infectious DiseaseLuis AlbertoNo ratings yet
- HHS Public Access: The Third International Consensus Definitions For Sepsis and Septic Shock (Sepsis-3)Document24 pagesHHS Public Access: The Third International Consensus Definitions For Sepsis and Septic Shock (Sepsis-3)graciasNo ratings yet
- Chronic Medical Conditions and Risk of SepsisDocument7 pagesChronic Medical Conditions and Risk of SepsisMargarita RamirezNo ratings yet
- KWF 113Document11 pagesKWF 113Residentes RadiologiaHCENo ratings yet
- Journal Pre-Proof: Journal of Cardiothoracic and Vascular AnesthesiaDocument36 pagesJournal Pre-Proof: Journal of Cardiothoracic and Vascular AnesthesiaBotez MartaNo ratings yet
- Molecular Dissection of Chagas Induced CardiomyopathyDocument31 pagesMolecular Dissection of Chagas Induced CardiomyopathyClaudia Calvet AlvarezNo ratings yet
- Tocilizumab en Covid 19Document12 pagesTocilizumab en Covid 19Frank Antony Arizabal GaldosNo ratings yet
- 10.5811@westjem.2020.6.47919 (2) 81105Document7 pages10.5811@westjem.2020.6.47919 (2) 81105Giovan GaulNo ratings yet
- Ruan2020 Article ClinicalPredictorsOfMortalityDDocument3 pagesRuan2020 Article ClinicalPredictorsOfMortalityDntnquynhproNo ratings yet
- The Natural History, Pathobiology, and Clinical Manifestations of Sars-Cov-2 InfectionsDocument28 pagesThe Natural History, Pathobiology, and Clinical Manifestations of Sars-Cov-2 InfectionsJosé Luis Alonso EscamillaNo ratings yet
- Hpaa 057Document2 pagesHpaa 057RISMA ARLYANINo ratings yet
- Emerging Infectious Disease Agents and Their Potential Threat To Transfusion Safety.Document29 pagesEmerging Infectious Disease Agents and Their Potential Threat To Transfusion Safety.Pritha BhuwapaksophonNo ratings yet
- Excellent Paper On Covid 19Document20 pagesExcellent Paper On Covid 19GalenNo ratings yet
- CrothersDocument8 pagesCrothersMETA INVESTIGACIÓN Y DESARROLLONo ratings yet
- NEJM. COVID CHINA. 10.1056@NEJMoa2002032Document13 pagesNEJM. COVID CHINA. 10.1056@NEJMoa2002032Boris CabreraNo ratings yet
- Jama Sanders 2020 RV 200005Document13 pagesJama Sanders 2020 RV 200005Benjamin Rodriguez CabreraNo ratings yet
- The Novel Coronavirus' Spike Protein Plays Additional Key Role in Illness - Salk Institute For Biological StudiesDocument4 pagesThe Novel Coronavirus' Spike Protein Plays Additional Key Role in Illness - Salk Institute For Biological StudieschingatchgookNo ratings yet
- Ali 01Document10 pagesAli 01Juan Fernando Garcia RobledoNo ratings yet
- COVID-19 and Multi-Organ ResponseDocument31 pagesCOVID-19 and Multi-Organ ResponseArbaz SultanNo ratings yet
- The Emergence and Persistence of C Auris in Western New YorkDocument4 pagesThe Emergence and Persistence of C Auris in Western New YorkLilia JouNo ratings yet
- BBA - Molecular Basis of Disease: ReviewDocument12 pagesBBA - Molecular Basis of Disease: ReviewautomationenggNo ratings yet
- Correspondence: Smell and Taste Dysfunction in Patients With COVID-19Document1 pageCorrespondence: Smell and Taste Dysfunction in Patients With COVID-19Dhyo Asy-shidiq HasNo ratings yet
- HHS Public Access: HypertensionDocument48 pagesHHS Public Access: HypertensionFarmasi RSUD Kramat JatiNo ratings yet
- A miRNA PEPTIDE FUSION AS A VACCINE CANDIDATE AGAINSTCOVID 19Document12 pagesA miRNA PEPTIDE FUSION AS A VACCINE CANDIDATE AGAINSTCOVID 19Pipirrin GonzalesNo ratings yet
- Prognostic Impact of Prior Heart Failure in Patients Hospitalized With COVID-19Document15 pagesPrognostic Impact of Prior Heart Failure in Patients Hospitalized With COVID-19Basilio BabarNo ratings yet
- Articulo Cientifico Derrame Pleural en Covid 19Document10 pagesArticulo Cientifico Derrame Pleural en Covid 19Leyla Barnard LagunaNo ratings yet
- Zhu2020 Article CardiovascularComplicationsInPDocument9 pagesZhu2020 Article CardiovascularComplicationsInPJRDEABREUNo ratings yet
- Jce 30035Document7 pagesJce 30035Sridevi DinakaranNo ratings yet
- Combing Through Traditional Texts To Prevent Covid-19 - A Scientific ApproachDocument14 pagesCombing Through Traditional Texts To Prevent Covid-19 - A Scientific ApproachVinayNo ratings yet
- DeSanto 3057Document8 pagesDeSanto 3057Maryka CociuNo ratings yet
- ABO Blood Type Association With SARS CoV 2 Infection Mortality 2021Document7 pagesABO Blood Type Association With SARS CoV 2 Infection Mortality 2021azizk83No ratings yet
- Raynaud’s Phenomenon: A Guide to Pathogenesis and TreatmentFrom EverandRaynaud’s Phenomenon: A Guide to Pathogenesis and TreatmentFredrick M. WigleyNo ratings yet
- Memo - COVID-19 Vaccine Distribution UpdateDocument7 pagesMemo - COVID-19 Vaccine Distribution UpdateCTV Ottawa100% (1)
- Office of The Punong Barangay Executive Order No. 03Document2 pagesOffice of The Punong Barangay Executive Order No. 03pablo gayodanNo ratings yet
- Dealing With Second Wave of Covid - 19Document11 pagesDealing With Second Wave of Covid - 19Sajith KumarNo ratings yet
- Activity Sheet Practical Research 1 Group 1Document28 pagesActivity Sheet Practical Research 1 Group 1SRHeart Nikcole Dela PazNo ratings yet
- Weekly CT-performance-monitoring-toolDocument1 pageWeekly CT-performance-monitoring-toolJurryNo ratings yet
- Waiver For Independent ContractorsDocument2 pagesWaiver For Independent ContractorsChristian Roque0% (1)
- Family Life Cycle Stage Major Task 2 Order Changes in Family Status Required To Proceed DevelopmentallyDocument6 pagesFamily Life Cycle Stage Major Task 2 Order Changes in Family Status Required To Proceed DevelopmentallyAris Jasper NacionNo ratings yet
- Renata 2019-2020Document194 pagesRenata 2019-2020Nayyab Alam TurjoNo ratings yet
- HOO - Coronavirus - Blanket - Isolation 7 1 20Document3 pagesHOO - Coronavirus - Blanket - Isolation 7 1 20mattbettNo ratings yet
- Acceptance and Attitude Toward Covid-19 Vaccination: A Cross-Sectional Study From Udaipur DistrictDocument8 pagesAcceptance and Attitude Toward Covid-19 Vaccination: A Cross-Sectional Study From Udaipur DistrictIJAR JOURNALNo ratings yet
- WB Covid Protocol Book 25.09 .20 (1)Document49 pagesWB Covid Protocol Book 25.09 .20 (1)El MirageNo ratings yet
- Acquired Immuno Deficiency Syndrome (Aids)Document9 pagesAcquired Immuno Deficiency Syndrome (Aids)alina20No ratings yet
- Effectiveness of Whatsapp For Blood Donor Mobilization Campaigns During Covid-19 PandemicDocument5 pagesEffectiveness of Whatsapp For Blood Donor Mobilization Campaigns During Covid-19 PandemicBala BhaskarNo ratings yet
- D Properties Covid 19 Self DeclarationDocument1 pageD Properties Covid 19 Self Declarationfahad atallaNo ratings yet
- Prevention and Treatment of COVID-19 in Elderly People: September 2020Document8 pagesPrevention and Treatment of COVID-19 in Elderly People: September 2020Dra. Adialys Guevara GonzalezNo ratings yet
- Camuso ResumeDocument2 pagesCamuso Resumeapi-548307464No ratings yet
- The Wall Street Journal - 07 04 2020 Optimize PDFDocument34 pagesThe Wall Street Journal - 07 04 2020 Optimize PDFMuhammad HafeezNo ratings yet
- 2.1 DF Non-Thai 7 JulyDocument3 pages2.1 DF Non-Thai 7 JulyJia YapNo ratings yet
- David and Limas EAPS Concept PaperDocument4 pagesDavid and Limas EAPS Concept Paperjepu jepNo ratings yet
- Makalah Bahasa Inggris Unit 6Document10 pagesMakalah Bahasa Inggris Unit 6Nadia NadNo ratings yet
- Letter From The Executive BoardDocument6 pagesLetter From The Executive BoardPratham AryaNo ratings yet
- Rapid Hospital Readiness ChecklistDocument19 pagesRapid Hospital Readiness ChecklistAnonymous KEU60hNo ratings yet
- Tema: Personal Information: Escuela Profesional de EnfermeríaDocument21 pagesTema: Personal Information: Escuela Profesional de EnfermeríaPamela Ballon SotoNo ratings yet
- JP Morgan COVID 19 Presentation PDF PDF PDF PDF PDF PDF PDF PDF PDFDocument45 pagesJP Morgan COVID 19 Presentation PDF PDF PDF PDF PDF PDF PDF PDF PDFFrancoNo ratings yet
- Reflect On The Significant Features of RA 11494.: Treatment of Covid-19 PatientsDocument3 pagesReflect On The Significant Features of RA 11494.: Treatment of Covid-19 PatientsGabriel FranciscoNo ratings yet
- Outpatient Otolaryngology in The Era of COVID-19: A Data-Driven Analysis of Practice PatternsDocument7 pagesOutpatient Otolaryngology in The Era of COVID-19: A Data-Driven Analysis of Practice PatternsMada Dwi HariNo ratings yet