Professional Documents
Culture Documents
HW 3 Orgo Chem I PDF
HW 3 Orgo Chem I PDF
Uploaded by
Madison Fuller0 ratings0% found this document useful (0 votes)
6 views9 pagesOriginal Title
HW 3 ORGO CHEM I.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views9 pagesHW 3 Orgo Chem I PDF
HW 3 Orgo Chem I PDF
Uploaded by
Madison FullerCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 9
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci., NFC, Madison, FL
HOME WORK — Il (CHAPTER-3), 15 points +5 bonus points to TEST-1
ORGANIC CHEMISTRY —1_ (CHM 2210), FALL 2020
Name & IDE (GAR Fallec Due date: Fri, Oct. 3, 2020
1) Show which pair of structures are identical, constitutional, resonance and non-identical.
i CAs erence
Ay yd Beseoane
ALL Watton
, sondiassi :
jumber the longest carbon chain to match the IUPAC name of each alkane below.
2)
See ot
aisobuy-2-metyoctane or aes
Atortbuyt2.7-dimettyiociane 4-etmy.2.5.6.8etametiyi7-propyidecane 4-0thy'3.6.7-timetryknonane
3) Apply IUPAC rules and provide a systematic name for each of the following alkanes, cycloalkanes
and bicycloalkanes. Numbering the chain or cycle is needed for each compound,
2
: te
3-elnut oe methyl hexane
2- clove ~ 3,5, U-benme Huylheptane
4 thors -3-e8
¥ —trwwecveyl-
seoorednglocteanie
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci., NFC, Madison, FL C+(3 ae an
ae ess
" 2.
4 e (Coy CHG nerseH(CHCTE)CHLCICHy)s . oe
NG ,2,E—teimetiyl ockane ne tN a
aa
HyCCHCH,CCH,CHCH, CH,
- i
CH O(CHad
2,2)5\3- Fe camne thy) 6 F -OMMO= 3 -ethyl 2255
+ eecea
tetramethylodte Ne
.\
B-ery! COR CH
crypt
Choe oH
1 -{soeeney) — L=mnesnuloctene
Bereta) —B, 4 dronetnyloctcige
WN 3,4 tA Slurs wane
Example: ChCH-CH;-CHaCI
1,1,3-trichloro propane.
Drawing Instructions: Draw skeletal strictures corresponding to each of the given names.
Draw: 6-ethyl-4-isopropyldecane .
oe
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci., NFC, Madison, FL
BC
3c
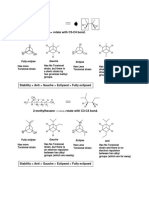
4) (I) Use Newman projection to Illustrate “four possible conformations of 2,3-dibromo-butane.
Eully Eclipsed Gauche
hs
sd ally 7
H Lo3
eg
wv 9 OC War ec
—aiaseae 2 - agg ae
i we lle Ay ‘
Gauche
Ectipsed |
Fully Eetipsed She Z
‘Decrease in the stability order Xe at i wf
Paks 7 Couche Zfstiose 7 lly ec tigseed — ite
(1) For2,3-dimethylbutane, draw the most stable “anti” form first and then accomplish both “gauche”
and “eclipsed” forms by using appropriate rotational (torsion) angle.
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci., NFC, Madison, FL
{lil) Obtain other conformations by clock-wise and counter clock-wise rotation for the following two
compounds (3,4-dimethylhexane and 2,3-dichlorobutane).
rv
4
8 300° W
Ht
A oe,
4
Belipsed - =
Woy ey
Hot
207 ots ize | 1 eh
« ens H CH
4 cng Gauche A
Jj 20 OM
ul
Uh,
y u
4 Hs
t
a
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci., NFC, Madison, FL
1) Draw Constitutional isomers of a cycloalkane with the M.F. CcH:2 and also name each of the isomer.
Osgh =
yelotoulane \ya-dometh,
I-enet r 'o v eo Aa L.Z-d
Cytiohexane Ni ley leReokne, Ayhcdsivet a) ble Bulo eT nome
Hyelolodude b
Vokes swety J
2,3 —Heyedhyleyelpragon
cg Latnoroea’ ne
H SICHs)s
a et
nycrn iH ‘5
OI ll lala
3) Draw cis-1-sec-butyl-2-ethyleyclopentane S's
Gem Lael
4) Draw 3,5-dieyclohexylnonane i
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci., NFC, Madison, FL.
5) Label each pair of compounds below as:
a. conformational isomers
b. stereoisomers
©. constitutional isomers
d. identical
sal eK
H H
6) Apply IUPAC rules and provide a systematic name for each of the following cycloalkanes and
icycloalkane:
Br br.
Ie ethyl—3-bepona -4-metha]
(-bome ao -ethg| Cgeloherane 4
( yotcherce ve
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci. NFC, Madison, FL
KS
\Apeoeno - 1,3,3-+ Timerdnyeylogentane Morome -3-et hayl A methileyeleoer
“ettid -3,4 -deme Huy legeloheplere Febhyl — fi -dimnetyleyelahicoteine
What's this name?
zl v
>
4
ob
3
re
1-(@-dnlero - cee Ge 44 -demetlyl 104 somone
Gpldeprane oes
ip pes n
Fncnicsnste Sagi 2 air
Servo [g.4] decor belo [asd] AoNAnE
\ A =
4 *
at ey 4 3
s ot i eee
Deeyelo (A. 2. aLundecane biewlo [s.4.0] Undecane
Bt 4 ae
(ae : 4 :
4 3 ei S
zi
3 at ee
Spice [uu] Loaded cane
drexelo U.4.6) dodecane?
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci., NFC, Madison, FL.
7) Mlustrate two possible chair forms of (i) trans-1-sec-butyl-3-ethyl-cyclohexane and (ii)
isobutyl-4-isopropyl-cyclohexane and circle the conformer that is more stable from each set,
cis-1-isobutyl-4-isopropyl-cyclohexane
ith aibae
8) (I) Circle which one is more stable from et pair of cha cavonnatishs and expen your, description
ina few words.
ke eB a 4 os : \
evwy\ vSequere sec Was Less (\3-dexa| gf
Steen,
Comecced o B vlowlalned mace 1,3 -deaxial shay: OAH due’ ko Phe Cray bony,
(w Complete the transformation of cyclohexane conformations. Also, assign the stereoisomer (cis/tlans)
of each pair of compounds. Wa ce eplecitacral pos fsa.
&
ws ¥ ws rea
Rinctar
Wann i
NT ee a = Ly
Instructor: Manoharan Mariappan, Ph.D., Dept of Nat. Sci., NFC, Madison, FL
9) Below are the two chair conformations of a 1,2,4-trimethylcyclohexane. Estimate the amount of 1,3
4lanal strain in each conformer and prediet which conformer is most stable.
}
bly B only en bS-chewe
i ey
cH: pee ON
A ae NRRL te
eg ei Croan Bee ei Wee eget
yeh Sis Xv
Nees hoo 13 -chexin)
Shey. Wns AK Cortgcernror :
‘b less Stedole clue $0 He
V-dheyial steer,
B ws We most sb conformer
10) On the templates provided, draw two chair conformations that are in equilibrium for Menthol as well
D-pinitol. Circle the mast stable conformation.
aw, M3 a
ieee
| J oN ec ° cH,
we “MoH Bs i 3
‘Menthol (in peppermint) 4 iM
QH
HCO, A OH Oy on "|
F oH
‘ > ou = Ho:
Ho" (OH ry a }
on
pinitol (antt-diabetc agent) ce SS a
You might also like
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Lab 4 Reduction of Camphor To IsoborneolDocument9 pagesLab 4 Reduction of Camphor To IsoborneolMadison FullerNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (346)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Even More Resonance Practice ProblemsDocument7 pagesEven More Resonance Practice ProblemsMadison FullerNo ratings yet
- Lab 1 Soluibility of Organic CompoundsDocument14 pagesLab 1 Soluibility of Organic CompoundsMadison FullerNo ratings yet
- Chap. 8 Reactions of Alkenes: (I) Electrophilic Addition ReactionsDocument9 pagesChap. 8 Reactions of Alkenes: (I) Electrophilic Addition ReactionsMadison FullerNo ratings yet
- Amines and AmidesDocument17 pagesAmines and AmidesMadison FullerNo ratings yet
- Organic Chemistry - I (CHM 2210), Fall 2020: TEST - I (Chapter 1 - 2), 100 Points + 10 BonusDocument6 pagesOrganic Chemistry - I (CHM 2210), Fall 2020: TEST - I (Chapter 1 - 2), 100 Points + 10 BonusMadison FullerNo ratings yet
- Butane - Rotate With C3-C4 Bond.: C H C H C H CH H H H HDocument11 pagesButane - Rotate With C3-C4 Bond.: C H C H C H CH H H H HMadison FullerNo ratings yet