Professional Documents
Culture Documents
Value of Omalizumab in Chronic Refractory Urticaria 2012 PDF
Uploaded by
Cristian Quito0 ratings0% found this document useful (0 votes)
5 views1 pageOriginal Title
value-of-omalizumab-in-chronic-refractory-urticaria-2012.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views1 pageValue of Omalizumab in Chronic Refractory Urticaria 2012 PDF
Uploaded by
Cristian QuitoCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
PD03—MEDICAL DERMATOLOGY Patient geography and relation to dermatology service utilization
(Poster reference number 5606)
Elaine Dupuis, University of British Columbia, Vancouver, Canada; Wingfield
Advancing pharmacovigilance through analysis of a gadolinium-based Rehmus, MD, MPH, University of British Columbia, Vancouver, Canada
contrast agent (GBCA) exposure dataset for nephrogenic systemic fibrosis:
A RADAR report Background: In many Canadian provinces, dermatologists are unevenly distributed.
Literature supports that patients in and near urban centers have the shortest travel
(Poster reference number 5325) distance to a dermatologist. Patients living at a great distance from a dermatologist
Beatrice Nardone, MD, PhD, Department of Dermatology, Northwestern might be referred less often for management of less severe skin problems because of
University, Chicago, IL, United States; Anne E. Laumann, MBChB, Department the cost of travel and relative inaccessibility of specialty care. This study set out to (a)
of Dermatology, Northwestern University, Chicago, IL, United States; Beatrice J. identify the top diagnoses seen at BC Children’s Hospital (BCCH) dermatology
Edwards, MD, MPH, Department of Medicine, Division of Geriatrics, clinic, (b) examine if diagnoses differ based on where the patient lives, and (c)
Northwestern University, Chicago, IL, United States; Dennis P. West, PhD, explore what relationship exists between distance from patient’s home to BCCH
Department of Dermatology, Northwestern University, Chicago, IL, United dermatology clinic and disease severity. We hypothesize that the further a patient
States; Steven M. Belknap, MD, Department of Dermatology, Northwestern travels to see a dermatologist, then the more severe their disease.
University, Chicago, IL, United States
Methods: A list of all new patient visits to BCCH dermatology clinic during 2009 was
Background: Nephrogenic systemic fibrosis (NSF) is a systemic fibrosing disorder compiled and sorted by ICD-10 codes to produce a ranking list of encountered
primarily affecting patients with end-stage renal disease (ESRD). Patients develop diagnoses. For each diagnosis, data were subsequently sorted by health region,
progressive, indurated plaques and confluent papules on their skin, visceral fibrosis, delivery area, and city of residence. Census data and definitions were used to classify
contractures, and may proceed to disability and elevated mortality. NSF is associated cases as urban or rural. For each health area, the most common reasons for referral to
with systemic exposure to gadolinium-based contrast agents (GBCAs). Higher GBCA dermatology were noted. For acne and atopic dermatitis cases, a retrospective chart
doses and/or cumulative dosing may raise the risk of NSF. review was undertaken to assess disease severity based on patient management.
Methods: RADAR (Research on Adverse Drug events And Reports) searched a GBCA- Patient demographics, primary reason for visit, prescribed treatment and follow-up
exposure dataset for NSF biopsy-proven cases, all with GBCA exposures and care were noted.
positively identified as linked to a specific product (good faith substantiation). Cases Results: The top 10 diagnoses at the BCCH dermatology clinic were: atopic
were characterized as unconfounded if it was determined that the patient was dermatitis (2102 cases), hemangioma (289), acne (263), psoriasis (145), warts
exposed to only one GBCA before NSF diagnosis and confounded if the patient was (121), congenital nevi (97), molluscum contagiosum (95), eczema, NEC (78), vitiligo
exposed to two or more GBCAs. The cases in this publicly available dataset are those (77), and alopecia areata (60). The most common reasons for referral to the
filed in federal court within the Gadolinium-Based Contrast Agents Product Liability dermatology clinic were comparable between health regions despite vast differ-
Litigation, MDL No. 1909. ences in geographic proximity to the clinic. The majority of patients were from
Results: A total of 382 individual biopsy-proven, product-specific NSF cases were urban areas. Only 0.1% of all cases for atopic dermatitis and 5.4% of all acne cases
analyzed. 76.1% (291/382) identified exposure to gadodiamide, of which 67.7% were from rural areas.
(197/291) were unconfounded. In addition, 40% (153/382) of cases involved Conclusion: The results suggest that the most frequent cases seen at BCCH
gadopentetate dimeglumine, of which 48.3% (74/153) were unconfounded while dermatology clinic are common diagnoses, even for patients who come from a
gadoversetamide was identified in 7.3% (28/382) of which 28.6% (8/28) were great distance to the clinic. This suggests that educational programs for primary care
uncounfounded. Some cases involved gadobenate dimeglumine or gadoteridol, 5.7% doctors in other areas in British Columbia should focus on management of these
(22/382) all of which were confounded. The mean number of exposures to GBCA common skin conditions. Complete results including chart review for disease
was gadodiamide (3), gadopentetate dimeglumine (5) and gadoversetamide (2). Of severity will be presented.
the 279 uncounfounded cases, all involved a linear-structured GBCA. Two hundred
and five (73.5%) were a nonionic GBCA while 74 (26.5%) were an ionic GBCA. Commercial support: None identified.
Conclusion: High GBCA dose, low patient renal function, presence of systemic
inflammation and chemical instability of the gadolinium-chelate are likely to be the
key determinants for NSF. Importantly, this publicly available legal dataset allows for
the identification of unconfounded cases with product-specific exposure
information.
Commercial support: None identified.
Value of omalizumab in chronic refractory urticaria
(Poster reference number 4738)
Miquel Armengot-Carbo, MD, Arnau de Vilanova Hospital, Valencia, Spain;
Beatriz Rodrigo-Nicolas, MD, Arnau de Vilanova Hospital, Valencia, Spain;
Enrique Gimeno Carpio, MD, Arnau de Vilanova Hospital, Valencia, Spain;
Manuel Velasco Pastor, MD, PhD, Arnau de Vilanova Hospital, Valencia, Spain;
Skin manifestations of outpatient adverse drug events in the United States: Virginia Pont Sanjuan, MD, Arnau de Vilanova Hospital, Valencia, Spain
A national analysis Background: Omalizumab is a monoclonal anti-IgE antibody approved for the
(Poster reference number 5560) treatment of severe allergic asthma. Several cases have been published in the
Peter Koelblinger, Wake Forest University School of Medicine, Winston Salem, literature, and there are some small series where it was used successfully for the
NC, United States; Scott Davis, Wake Forest University School of Medicine, treatment of both autoimmune and nonautoimmune chronic urticaria (CU).
Winston Salem, NC, United States; Steve Feldman, MD, PhD, Wake Forest Purpose: We performed a review of the patients treated in our department with
University School of Medicine, Winston Salem, NC, United States; Tushar omalizumab for the purpose of evaluating its efficacy in refractory CU.
Dabade, MD, Wake Forest University School of Medicine, Winston Salem, NC,
United States Methods: A retrospective case review was performed over 15 patients with CU that
started treatment with omalizumab during the year 2010. Their medical records
Background: Cutaneous reactions to drugs are among the most common clinical were reviewed to evaluate the improvement obtained at 3 and 6 months of
manifestation of adverse drug events (ADEs); however, data on outpatient cutaneous treatment. For this retrospective assessment, the response obtained was classified as
adverse drug events (CADEs) are limited. follows: lack of response, mild response (symptom improvement with no reduction
Purpose: To provide national estimates of outpatient CADEs and determine their of previous treatments), moderate response (symptom improvement with reduc-
most frequent causes. tion of previous treatments), and complete response (asymptomatic with no other
Methods: Outpatient CADEs recorded in the National Ambulatory Medical Care added treatment).
Survey (NAMCS) and the National Hospital and Ambulatory Medical Care Survey Results: The mean duration of the clinical symptoms was 6 years (range, 6 months to
(NHAMCS) between 1995 and 2005 were analyzed. By applying sampling weights, 22 years). All patients were refractory to antihistamines and some were also to
the national annual incidence of outpatient CADEs in the United States was corticoids and/or cyclosporine. After 3 months of treatment, 12 of the 15 patients
estimated and the most frequent medication classes implicated with CADEs were (80%) obtained some type of response. Three patients (20%) obtained a complete
identified. response, 7 (47%) a moderate response, 2 (13%) a mild response, and 3 (20%) had no
Results: Our analysis yielded a mean annual total of 635,982 CADE-related visits, response. Of nonresponders, two discontinued treatment; the dose was increased in
resulting in an annual incidence of 2.26 CADEs per 1,000 persons. Patients took an one; and one patient with moderate response discontinued for work reasons. The
average of 2.2 medications in addition to the one causing the CADE. The incidence drug was removed in two patients with complete response and they experienced
of CADEs increased with age, with a peak in the age group from 70-79 years. The clinical relapse after 5 weeks, so omalizumab was restarted, obtaining again a
medications most frequently causing a CADE were antimicrobial agents, with complete response (persistent after 2 months follow-up). Six-month data are
amoxicillin being the most frequent single causative substance. Dermatitis and available for 10 patients: eight had obtained or maintained a complete response,
urticaria were the two main types of skin reactions reported. Limitations: Estimates and two had a mild response. None of those obtaining response at 3 months lost
were limited by the sample size of 539 visit records. Conclusions: CADEs occur less efficacy at 6 months.
frequently in outpatients than for inpatients, and result in fewer hospital admissions Conclusion: Although further studies supporting it are required, the results obtained
than ADEs in general (1.2 vs 2.5%). Physicians must be particularly cognizant of the in our series show that omalizumab is an effective therapeutic tool in cases of
occurrence of CADEs when prescribing antimicrobial agents. chronic urticaria not responding to conventional treatments.
Commercial support: None identified. Commercial support: None identified.
APRIL 2012 J AM ACAD DERMATOL AB5
You might also like
- Visceral Zoster As The Presenting Feature of Disseminated Herpes ZosterDocument4 pagesVisceral Zoster As The Presenting Feature of Disseminated Herpes ZosterCristian QuitoNo ratings yet
- Polymorphic Eruption of Pregnancy: ReviewDocument7 pagesPolymorphic Eruption of Pregnancy: ReviewCristian QuitoNo ratings yet
- Psoriasis Lesions Are Associated With Specific Types of Emotions. Emotional Profile in PsoriasisDocument6 pagesPsoriasis Lesions Are Associated With Specific Types of Emotions. Emotional Profile in PsoriasisCristian QuitoNo ratings yet
- A Case-Control Study of Polymorphic Eruption of Pregnancy: A B C B ADocument5 pagesA Case-Control Study of Polymorphic Eruption of Pregnancy: A B C B ACristian QuitoNo ratings yet
- Neurological and Psychiatric Disorders in Psoriasis 2018Document9 pagesNeurological and Psychiatric Disorders in Psoriasis 2018Cristian QuitoNo ratings yet
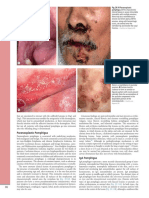
- Fig. 29.10 Paraneoplastic Pemphigus. A The CharacteristicDocument1 pageFig. 29.10 Paraneoplastic Pemphigus. A The CharacteristicCristian QuitoNo ratings yet
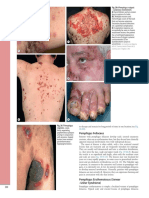
- Fig. 29.6 Pemphigus Vulgaris - Cutaneous Involvement. A Flaccid Blisters and An Erosion B, C Multiple Erosions andDocument1 pageFig. 29.6 Pemphigus Vulgaris - Cutaneous Involvement. A Flaccid Blisters and An Erosion B, C Multiple Erosions andCristian QuitoNo ratings yet
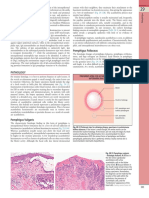
- Immunologic Mechanism of Pathogenic Autoantibody Production in PemphigusDocument1 pageImmunologic Mechanism of Pathogenic Autoantibody Production in PemphigusCristian QuitoNo ratings yet
- Pemphigus Foliaceus: Fig. 29.13BDocument1 pagePemphigus Foliaceus: Fig. 29.13BCristian QuitoNo ratings yet
- Herpetiform Pemphigus: Courtesy, Ronald P Rapini, MDDocument1 pageHerpetiform Pemphigus: Courtesy, Ronald P Rapini, MDCristian QuitoNo ratings yet
- JA A D: M CAD ErmatolDocument2 pagesJA A D: M CAD ErmatolCristian QuitoNo ratings yet
- Antifungal Agents: LipopeptidesDocument8 pagesAntifungal Agents: LipopeptidesCristian QuitoNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Management of Asthma During Pregnancy: Symposium ContDocument6 pagesManagement of Asthma During Pregnancy: Symposium ContWulan CerankNo ratings yet
- Vitamin D Treatment For Chronic Urticaria A Case RDocument3 pagesVitamin D Treatment For Chronic Urticaria A Case RSisiliana KristinNo ratings yet
- Biotech Drugs - Biological Therapeutic Agents PDFDocument4 pagesBiotech Drugs - Biological Therapeutic Agents PDFRenan Vieira Zaffanelli SallesNo ratings yet
- ASTHMA - WatermarkedDocument7 pagesASTHMA - WatermarkedShubhangiNo ratings yet
- Asthma and Other Respiratory Medications DefindDocument4 pagesAsthma and Other Respiratory Medications DefindKiran ManojNo ratings yet
- Alergy 2Document2 pagesAlergy 2Wiwiek Anindita AfrilyantariNo ratings yet
- Anaphylaxis in Children and Adults Prevention, Diagnosis and TreatmentDocument88 pagesAnaphylaxis in Children and Adults Prevention, Diagnosis and TreatmentMichael DiPrimaNo ratings yet
- Anaphylactic Shock - Pathophysiology, Recognition, and Treatment PDFDocument9 pagesAnaphylactic Shock - Pathophysiology, Recognition, and Treatment PDFTita Luthfia100% (1)
- New Treatments For Severe Treatment-Resistant Asthma - Lancet Resp Med 2013Document14 pagesNew Treatments For Severe Treatment-Resistant Asthma - Lancet Resp Med 2013Azan Al RasyidNo ratings yet
- Ghid Astm BronsicDocument11 pagesGhid Astm BronsicLeonard D100% (1)
- Chemotherapy and Biotherapeutic Agents For Autoimmune DiseasesDocument17 pagesChemotherapy and Biotherapeutic Agents For Autoimmune DiseasesIriani Dewi SetiawanNo ratings yet
- Arun Selvaratnam Resume 1Document3 pagesArun Selvaratnam Resume 1api-341631469No ratings yet
- Severe Asthma: Definition, Diagnosis and TreatmentDocument9 pagesSevere Asthma: Definition, Diagnosis and TreatmentHalim Muhammad SatriaNo ratings yet
- Asthma Case StudyDocument39 pagesAsthma Case StudyDimitris TasiouNo ratings yet
- Xolair Dosing GuideDocument12 pagesXolair Dosing Guidemohamed muhsinNo ratings yet
- 06 - Ambulatory Care PDFDocument50 pages06 - Ambulatory Care PDFandirio7486No ratings yet
- Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020Document13 pagesUrticaria: Collegium Internationale Allergologicum (CIA) Update 2020Ihdinal MuktiNo ratings yet
- The Relationship Between IgE and Allergic DiseaseDocument12 pagesThe Relationship Between IgE and Allergic DiseaseAbdusSomadNo ratings yet
- Urticaria and Angioedema - An Update On Classification and Pathogenesis PDFDocument14 pagesUrticaria and Angioedema - An Update On Classification and Pathogenesis PDFancillaagraynNo ratings yet
- 1991 - de Groot Et Al. - Affinity Purification of A Major and A Minor Allergen From Dog Extract Serologic Activity of Affinity-Purified CanDocument10 pages1991 - de Groot Et Al. - Affinity Purification of A Major and A Minor Allergen From Dog Extract Serologic Activity of Affinity-Purified Canpond_1993No ratings yet
- Concise Clinical Review: Role of Biologics in AsthmaDocument13 pagesConcise Clinical Review: Role of Biologics in AsthmaHossam HamzaNo ratings yet
- New Biologics in The Treatment of UrticariaDocument7 pagesNew Biologics in The Treatment of UrticariaArezoo JamNo ratings yet
- The Therapeutic Monoclonal Antibody MarketDocument7 pagesThe Therapeutic Monoclonal Antibody MarkethzluyuanNo ratings yet
- Biologic Therapies For Severe Asthma 2022Document15 pagesBiologic Therapies For Severe Asthma 2022Marina DomencoNo ratings yet
- (13142143 - Folia Medica) Pharmacoeconomics of Bronchial AsthmaDocument9 pages(13142143 - Folia Medica) Pharmacoeconomics of Bronchial AsthmaChristinaNo ratings yet
- 12.hypersensitivity 2013Document58 pages12.hypersensitivity 2013lookmoo100% (1)
- The Pathophysiology - Diagnosis and Treatment of Allergic RhinitisDocument16 pagesThe Pathophysiology - Diagnosis and Treatment of Allergic RhinitisAnonymous XFDJfsGviNo ratings yet
- Pediatric Chronic Sinusitis Diagnosis and ManagementDocument10 pagesPediatric Chronic Sinusitis Diagnosis and ManagementcaromoradNo ratings yet
- Omalizumab - Drug Information - UpToDate-2Document5 pagesOmalizumab - Drug Information - UpToDate-2Vh TRNo ratings yet
- The Production of Biopharmaceuticals: B. Hughes, L.E. HannDocument2 pagesThe Production of Biopharmaceuticals: B. Hughes, L.E. HannEE KMNo ratings yet