0% found this document useful (0 votes)
2K views17 pagesColligative Properties Power Point Presentation
Colligative properties depend on the ratio of solute particles to solvent molecules, not on the chemical species. There are four main types: lowering of vapor pressure, elevation of boiling point, depression of freezing point, and osmosis/osmotic pressure. These properties can be used to determine the abnormal molar mass of electrolyte solutions based on their degree of dissociation or association, represented by the van't Hoff factor.
Uploaded by
Chetan UpadhyayCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
2K views17 pagesColligative Properties Power Point Presentation
Colligative properties depend on the ratio of solute particles to solvent molecules, not on the chemical species. There are four main types: lowering of vapor pressure, elevation of boiling point, depression of freezing point, and osmosis/osmotic pressure. These properties can be used to determine the abnormal molar mass of electrolyte solutions based on their degree of dissociation or association, represented by the van't Hoff factor.
Uploaded by
Chetan UpadhyayCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Colligative Properties Overview: Provides an overview of colligative properties and their dependence on solute concentration.
- Relative Lowering of Vapour Pressure: Discusses the principle of vapour pressure lowering in solutions and explains Raoult's law with examples.
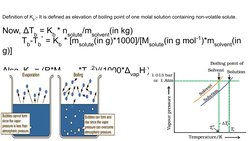
- Elevation of Boiling Point: Explains how boiling point elevation occurs in solutions and provides formulae for calculation.
- Depression of Freezing Point: Describes the depression of freezing point in solutions and its practical applications.
- Osmosis and Osmotic Pressure: Covers the principles of osmosis, osmotic pressure, and their relation to solution concentration.
- Abnormal Molar Mass: Defines conditions under which abnormal molar mass is observed and explains related calculations.