Professional Documents
Culture Documents
Aratow M Et Al 1993
Uploaded by
blaiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aratow M Et Al 1993
Uploaded by
blaiCopyright:
Available Formats
Intramuscular pressure and electromyography as indexes
of force during isokinetic exercise
M. ARATOW, R. E. BALLARD, A. G. CRENSHAW, J. STYF, D. E. WATENPAUGH,
N. J. KAHAN, AND A. R. HARGENS
Life Science Division, National Aeronautics and Space Administration Ames Research Center,
Moffett Field, California 94035-l 000
ARATOW, M.,R. E. BALLARD, A. G. CRENSHAW,J. STYF, robotics research. The problem of muscle atrophy in as-
D.E. WATENPAUGH,N. J. KAHAN,AND A.R. HARGENS.Intra- tronauts during long-term exposure to microgravity (23)
muscular pressure and electromyography as indexes of force dur- may be resolved through the development of effective
ing isokinetic exercise. J. Appl. Physiol. 74(6): 2634-2640, 1993. and efficient exercise hardware and protocols. One key to
-A direct method for measuring force production of specific understanding the time course of and preventing
muscles during dynamic exercise is presently unavailable. Pre-
vious studies indicate that both intramuscular pressure (IMP) strength loss due to muscle atrophy is the assessment of
and electromyography (EMG) correlate linearly with muscle joint torques generated by individual muscles during ex-
contraction force during isometric exercise. The objective of ercise.
this study was to compare IMP and EMG as linear assessors of Electromyography (EMG) has been used extensively
muscle contraction force during dynamic exercise. IMP and as a technique for monitoring force output during isomet-
surface EMG activity were recorded during concentric and ec- ric contractions. Over the past 40 years, however, inter-
centric isokinetic plantarflexion and dorsiflexion of the ankle pretation of data obtained using this technique has re-
joint from the tibialis anterior (TA) and soleus (SOL) muscles mained controversial. Although many investigations
of nine male volunteers (28-54 yr). Ankle torque was measured have reported a linear relationship between isometric
using a dynamometer, and IMP was measured via catheteriza-
tion. IMP exhibited better linear correlation than EMG with force and EMG, particularly at submaximal contraction
ankle joint torque during concentric contractions of the SOL levels (10, 12, 17), an almost equal number have found
(IMP R2 = 0.97, EMG R2 = 0.81) and the TA (IMP R2 = 0.97, this relationship to be nonlinear (14,17,20). The linear-
EMG R2 F 0.90), as well as during eccentric contractions (SOL: ity of EMG-force relationships may be highly muscle de-
IMP R2 = 0.91, EMG R2 = 0.51; TA: IMP R2 = 0.94, EMG R2 = pendent (14). Differences in muscle fiber type, recruit-
0.73). IMP provides a better index of muscle contraction force ment pattern, and firing frequency affect the linearity of
than EMG during concentric and eccentric exercise through isometric EMG-force relationships (20). The use of EMG
the entire range of torque. IMP reflects intrinsic mechanical as an indicator of contractile force during nonisometric
properties of individual muscles, such as length-tension rela- exercise poses additional problems. EMG is insensitive
tionships, which EMG is unable to assess. to changes in fiber length and contraction velocity (lo),
factors that significantly affect overall force production
eccentric; concentric; muscle force; biomechanical models of a dynamically contracting muscle. EMG represents
electrical excitation of muscle rather than its intrinsic
mechanical properties and may therefore be unsuitable
CURRENTLY, NO DIRECT practical method exists for mea- for monitoring specific muscle function during dynamic
suring force production of individual muscles during dy- exercise.
namic exercise in humans. Implantation of a buckle Intramuscular pressure (IMP), the fluid pressure cre-
transducer on a tendon is the most direct technique for ated by a muscle as it contracts within its fascial com-
individual muscle force assessment (ll), but it is highly partment, correlates linearly with contraction force in
invasive and impractical for regular use. Measurements specific muscles during isometric exercise over a wide
of dynamic torque over a joint are inadequate for deter- range of force (16, 18, 19). Although the magnitude of
mining contraction force of individual muscles, because IMP varies with catheter position and depth (19), corre-
several muscles often contribute to torque development. lation of IMP with isometric contraction force is consis-
Knowledge of individual muscle force production dur- tently linear. At present, however, there are no data avail-
ing dynamic exercise could benefit various clinical and able concerning the simultaneous measurement of IMP
research areas. Development of devices and protocols for and muscle contraction force during dynamic exercise.
rehabilitation and athletic training of specific muscles The purpose of this investigation was to determine
will be possible with this information. The ability to whether EMG or IMP correlates linearly with ankle joint
monitor individual muscles during dynamic exercise will torque during both concentric and eccentric exercise of
be useful for gait analysis and validation of biomechani; the tibialis anterior and soleus muscles in humans. We
cal models of joint systems (13). In addition, knowledge hypothesized that IMP would provide a more linear in-
of activation patterns and force contributions of specific dex of ankle joint torque (and theoretically of muscle
muscles may prove valuable for muscle pathology and contraction force) than EMG during dynamic exercise.
2634
Downloaded from journals.physiology.org/journal/jappl (080.030.208.074) on August 12, 2020.
IMP AND EMG DURING ISOKINETIC EXERCISE 2635
METHODS Each fluid-filled catheter was connected via 60 cm of
pressure tubing to a microcapillary infusion system set at
IMP and surface EMG activity were measured continu- a constant infusion rate of 1.0 ml/h (21).
ously and simultaneously in the tibialis anterior and so- Exercise apparatus and protocol. To familiarize the
leus muscles of nine male volunteers (age 28-54 yr). The subject with the dynamometer and protocol, two practice
protocol was approved by the Human Research Experi- sessions on two separate occasions were performed be-
ments Review Board at National Aeronautics and Space fore the data collection test date. These sessions con-
Administration Ames Research Center. All subjects gave sisted of the complete protocol without catheter inser-
informed written consent and were in excellent health, as tion or application of EMG electrodes.
determined by a physical examination and laboratory A Lido Active isokinetic dynamometer (Loredan Bio-
testing. IMP and EMG were measured at rest, during medical, Davis, CA) was used to guide the exercise proto-
passive movement, and during isometric, concentric, and col and to measure joint angle and torque. Before begin-
eccentric exercises. In addition, ankle torque and posi- ning the exercises, all subjects performed warm-up and
tion were measured continuously and simultaneously. stretching maneuvers of the soleus and tibialis anterior
For each subject, the leg to be studied was randomly pre- for 5 min. After the warm-up period, the catheters were
determined. inserted and EMG electrodes were placed on the desig-
IMP catheter and EMG electrode placement. IMP was nated leg. With the foot relaxed on the footplate of the
measured using a 16-gauge Teflon catheter with four side dynamometer and the knee positioned at 90° of flexion,
holes at the tip (Myopress, Atos Medical, Horby, Swe- the neutral position (0”) for each subject was set at a 90°
den) (21). With the subject in a prone position, the distal angle between the foot and the tibia (Fig. 1). A pilot study
soleus region was shaved and sterilized with providone- in our laboratory showed no EMG activity or IMP
iodine, draped sterilely, and anesthetized with 2-3 ml of changes in the gastrocnemius muscle with the soleus
2% lidocaine solution. The insertion point was on the muscle actively contracting and the knee positioned at
posterolateral aspect of the leg one-third of the distance 90° flexion.
between the lateral malleolus and the lateral tibia1 con- While the subject was still relaxed, the footplate was
dyle. A 5.7-cm 16-gauge trocar with a plastic sheath moved to the maximum dorsiflexion position (22 t lo
(Jelco, Critikon, Tampa, FL) was used to place the Myo- dorsiflexed from neutral) and the maximum plantarflex-
press catheter into the muscle in a proximal direction ion position (30 t 2’ plantarflexed from neutral) as de-
and at an acute angle (--30”) to the skin surface. After termined by the subject, and these positions were stored
penetration of the muscle fascia, the trocar was with- as limits of footplate motion. Torques generated by the
drawn ~2.5 mm into the sheath, and the sheath was ankle due to gravity and stretch of muscle components
bluntly advanced in a direction parallel with the muscle during passive movement through this range were stored
fibers to a depth of ~2.5 cm. The trocar was then re- and automatically subtracted by the dynamometer sys-
moved, and the catheter was inserted through the sheath. tem from subsequent active torques to give a net torque
At the point where the catheter contacted the muscle due to muscle contraction alone.
(resistance noted), the sheath was withdrawn from Exercise began with soleus isometric maximal volun-
around the catheter. The catheter was secured to the tary contraction (MVC) three times and then 75,50, and
skin with Steristrips and nylon tape. Catheter placement 25% MVC (twice at each level), as guided by a graphic
in the soleus muscle was confirmed by pressure pulses representation of joint torque on a computer monitor
during palpation and active plantarflexion of the foot driven by the dynamometer. Ankle position for isometric
with the knee bent to 90’ to minimize gastrocnemius exercises was maintained at the defined 0’ neutral posi-
contribution to plantarflexion torque. The length of tion. An identical series of tibialis anterior isometric con-
catheter inserted through the skin was 5.7 t 0.9 (SE) cm. tractions was subsequently performed.
A surface EMG electrode measuring 1 cm in diameter After 23 min of rest to avoid fatigue, the subject was
was placed on the skin directly above the location of the asked to contract the soleus concentrically from neutral
catheter tip. A reference electrode was placed ~4 cm position to maximum plantarflexion against resistance
distal to the active electrode, and a ground lead was supplied by the dynamometer, such that angular velocity
placed over the tibia. The skin under all surface elec- was maintained at 15”/s. A total of five consecutive con-
trodes was abraded with Redux paste (Hewlett-Packard, centric isokinetic soleus contractions were performed.
Palo Alto, CA) to minimize surface impedance (avg sur- The subject was encouraged to use maximal effort for
face impedance 11 t 3 kQ for soleus and-17 t 7 kQ for each contraction. An identical series of concentric tibialis
tibialis anterior). anterior contractions was subsequently performed start-
The subject was then placed in the supine position, and ing from the ankle neutral position.
the tibialis anterior catheter and EMG electrodes were The final portion of the protocol involved eccentric
similarly placed. The catheter insertion point for the ti- contractions against the dynamometer footplate, moving
bialis anterior was 3 cm distal and 1 cm lateral to the at an angular velocity of l5”/s. Beginning with the ankle
tibia1 tuberosity, with the catheter directed distally at an in the fully plantarflexed position, the subject resisted
acute angle (-20”) to the skin. Tibialis anterior cath- the movement of the footplate back to the neutral posi-
eters were placed an average length of 7.7 t 0.5 cm tion for five consecutive soleus eccentric contractions.
through the skin. Catheter placement in the tibialis ante- An identical series of tibialis anterior eccentric contrac-
rior was confirmed by pressure pulses during palpation tions was performed with the foot beginning in the fully
and active dorsiflexion of the foot. dorsiflexed position.
Downloaded from journals.physiology.org/journal/jappl (080.030.208.074) on August 12, 2020.
2636 IMP AND EMG DURING ISOKINETIC EXERCISE
Surface
EMG
IMP
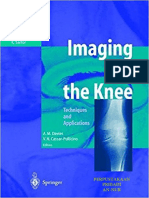
FIG. 1. Experimental setup. Knee
joint of designated leg was positioned at
IMP 90” of flexion throughout study. Ankle
neutral position (0”) was defined as 90”
angle between foot and tibia. IMP, intra-
Surface muscular pressure.
EMG
Lido Active controller
Data acquisition and display. IMP was measured by were calculated. Arcsine transformations were per-
precalibrated pressure transducers (model MS-20, Elec- formed on the mean R2 values, and the Kolmogorov-
tromedics, Austin, TX). The EMG signals were collected, Smirnov procedure was >used to test for normality (24).
amplified, and processed to produce root mean square Paired t/tests were used to delineate significant differ-
values by a four-channel EMG system (model 7400, Cad- ences between IMP and EMG R2 values. P < 0.05 was
well, Kennewick, WA). Preamplifier input impedance considered significant. Data are means t SE. Tibialis
was 10 MQ. Bandwidth was set at lo-1,000 Hz, with a anterior EMG was measured in eight subjects, and tech-
signal gain of 100 and lo-Hz filtering. Ankle torque and nical difficulties led to exclusion of concentric soleus
position measurements were provided by the Lido Active EMG data from one subject.
system. All data were collected and displayed in real time
on a chart recorder (model MT-9500, Astromed, W. RESULTS
Warwick, RI). In addition, the data were simultaneously
acquired by anJBM XT-compatible computer with use For all cases of dynamic exercise in this study, R2 val-
of LabTech Notebook software (Laboratory Technolo- ues for IMP vs. torque were significantly greater than R2
gies, Wilmington, MA) to drive a data acquisition board values for EMG vs. torque, as determined by paired t
(DAS 20, Metrabyte, Taunton, MA) at a rate of 8 Hz/ tests (P < 0.01). The contribution of passive stretch to
channel. IMP in both the soleus and tibialis anterior was negligi-
Statistical analyses. Only one contraction was selected ble in the ankle ranges used (1.0 t 0.5 mmHg for plantar-
for data analysis from each series: the contraction that flexion and 2.5 t 0.5 mmHg for dorsiflexion).
displayed the greatest torque was selected in the case of Isometric contractions. Both IMP and EMG correlated
the 100% MVC exercises (isometric, concentric, and ec- linearly with ankle joint torque in the soleus and tibialis
centric), and the contraction that most closely matched anterior of all subjects (Fig. 2). Linear regression analy-
the targeted level of torque was selected for the 75, 50, sis of soleus IMP and EMG variations with torque
and 25% MVC isometric exercises. This helped ensure yielded mean R2 values of 0.93 t 0.02 and 0.96 t 0.01,
that the MVC was used for all subjects and helped avoid respectively. Similarly, mean R2 values for tibialis ante-
inclusion of contractions in which fatigue may have been rior IMP and EMG against force were 0.96 t 0.01 and
a factor. 0.96 t 0.02, respectively.
Because of the wide range of peak torques generated Concentric contraction. Figure 3A shows one subject’s
by the different subjects due to their varied levels of mus- original strip chart tracings of torque, EMG, and IMP
cle mass and condition, torque is presented as percentage during a concentric contraction of the soleus. Both IMP
of maximum for each contraction (O-100%). EMG values and EMG appear to follow patterns similar to torque.
were normalized to a percentage of the maximum ob- Linear regression analysis of soleus IMP and EMG
served EMG value in each subject (17). Individual linear against torque during concentric contraction yielded
regression analyses of soleus and tibialis anterior IMP mean R2 values of 0.97 t 0.01 and 0.81 t 0.04, respec-
and EMG variations with torque were performed for tively (Fig. 4). The mean R2 values for tibialis anterior
each subject, and mean coefficients of determination (R2) IMP and EMG vs. torque during concentric contraction
Downloaded from journals.physiology.org/journal/jappl (080.030.208.074) on August 12, 2020.
IMP AND EMG DURING ISOKINETIC EXERCISE 2637
EMG also correlate well, but not as well as IMP, with
joint torque in most subjects during concentric exercise.
Although all linear regression R2values were significant
mean R2 = 0.93f 0.02 (indicating that both parameters correlated linearly with
joint torque), EMG-force relationships during eccentric
exercise were clearly nonlinear, reaching plateau values
at relatively low levels of torque. Isometric exercises per-
formed at various loads in our study confirm previous
findings that both IMP and EMG correlate well with
force during isometric exercise (13, 16, 19).
A
Particularly during eccentric exercise, EMG seems to
mean R2 = 0.96 +, 0.01 follow a more inverse exponential than linear pattern
I I I I I with force. For example, the inverse exponential equa-
tion y = 93.5(1 - e-o*o58X)can be fit to the EMG data in
Fig. 7 to give a mean R2 of 0.97 t 0.01. Using this type of
nonlinear equation to assess force would be extremely
mean R2 = 0.96 f 0.01 difficult, because EMG values level off at ~30% of maxi-
a mal torque. Also, because of intermuscle and possibly
f intersubject differences in EMG-force profiles (14,20), a
separate curve would need to be determined for each
a muscle under study.
f Figure 3 helps explain the nonlinearity of EMG during
an eccentric contraction. During the concentric contrac-
tion, peak force is reached relatively early in the contrac-
mean R 2 = 0.96 zk 0.02
tion, and both IMP and EMG increase with it. During the
0A 1 eccentric contraction, however, the peak force is greater
I I I I in magnitude and is reached late in the contraction (near
0 25 50 75 100
the limit of ankle dorsiflexion). Before peak eccentric
torque (% max)
FIG. 2. Soleus ( l ) and tibialis anterior (A) IMP and EMG vs. ankle
joint torque during isometric contractions of 25, 50, 75, and 100% of
maximal voluntary contraction (MVC). Error bars, SE. Individual lin-
ear regression analyses were performed for each subject, and mean R2
values were calculated. n = 8 for tibialis anterior EMG; all others n = 9.
were 0.97 t 0.01 and 0.90 t 0.03, respectively (Fig. 5). I
500
Maximum concentric torque for the nine subjects aver- I
aged 74 t 7 N m for the soleus and 34 t 3 N m for the
l l
I
tibialis anterior. 400 400
Eccentric contraction. Figure 3B shows one subject’s I
original strip ‘chart tracings of torque, EMG, and IMP I I
I y I
during an eccentric contraction of the soleus. IMP fol- 300 I 300 : ~ I
lows a pattern similar to torque, whereas EMG levels off I
I
early in the contraction. I
During eccentric contraction, IMP in the soleus corre- 200 I EMG (pV) 200
lated well with torque (R2 = 0.9i t 0.02), whereas EMG I
I
correlated poorly (R2 = 0.51 t 0.08; Fig. 6). Mean R2 val- I
ues for tibialis anterior IMP and EMG vs. torque during 100 I 100
I
eccentric contraction were 0.94 t 0.02 and 0.73 t 0.03,
respectively (Fig. 7). Maximum eccentric torque for the
nine subjects averaged 125 5 10 N. m for the soleus and 0: -
48 t 3 N. m for the tibialis anterior. For both muscles, 200 200
r
maximum eccentric torques were significantly greater
than maximum concentric and isometric torques. I IMP (mmkig)
100
n I
100
OPls ou
DISCUSSION 1 t
These findings convincingly demonstrate that IMP 0 12 3 4 0 1 2'3 4
provides a more linear index of contraction force than Time (s) Time (s)
EMG for the soleus and tibialis anterior during concen-
FIG. 3. Strip chart record of torque, EMG, and IMP for 1 subj dur-
tric and eccentric contractions. IMP values in both mus- ing a concentric (A) and an eccentric (B) contraction of soleus. Dashed
cles correlate linearly with joint torque during concentric line, time of peak torque. Ankle position ranged between 0 and 36O of
as well as eccentric exercise. Soleus and tibialis anterior plantar flexion.
Downloaded from journals.physiology.org/journal/jappl (080.030.208.074) on August 12, 2020.
2638 IMP AND EMG DURING ISOKINETIC EXERCISE
A force during contractions near the upper limit of muscle
mean R* = 0.97f0.01 length. Because motor units are not activated to generate
this tension, EMG cannot measure it. Contributions of
passive tension to total muscle contraction force in this
study were minimal, because the defined neutral position
did not allow either muscle under study to reach its max-
imum length during contraction.
Our findings confirm that maximum eccentric muscle
activation generates greater force than maximum con-
centric muscle activation (1,3,22). Although the explana-
tion for higher eccentric forces is unclear, various inves-
tigators have proposed biochemical (5), biomechanical
(2,4), and neurophysiological(l0) mechanisms. Regard-
less of the mechanism behind the increased strength of
‘d100 eccentric muscle activation, the fact that IMP changes
result from, rather than initiate or maintain, a contrac-
c tion indicates that IMP measurement may be a better
CJ
E 80 technique than EMG for monitoring muscle contraction
force during dynamic exercise.
W
60 In previous studies, investigators often chose intramus-
z cular electrodes to measure EMG for comparison with
N
; 40 force or IMP (12, 13, 17). More recent studies have
shown, however, that surface electrodes give a more accu-
E
rate and reproducible measure of activity of superficial
'0 20
c muscles (8,9,15). Intramuscular EMG electrodes record
the activity of only a few motor units in the immediate
o? . ’ . ’ - ’ - ’ - ’ ’ vicinity of the electrode. Because IMP >30 mmHg can
0 20 40 60 80 100 restrict blood flow in certain areas of skeletal muscle and
torque (% max)
FIG. 4. Soleus IMP (A; n = 9) and EMG (B; n = 8) during concentric
exercise. Each line represents 1 MVC from 1 subj up to maximum
analysis of each line yielded an R2value, and
A
torque. Linear regression mean R* = 0.97 + 0.01
mean + SE of all R2values is shown.
force is reached, EMG has already reached a plateau.
Beyond this point, factors other than fiber recruitment
and firing frequency are contributing to the overall force
output.
Although muscle fatigue is often cited as a reason for
the nonlinearity of EMG in relation to contraction force
at maximal isometric work loads, this is probably not the
case in our protocol, which was designed to avoid fatigue.
Instead, the known length-tension properties of skeletal
muscle probably explain, at least in part, why IMP may
relate better to muscle force production than EMG dur-
ing isokinetic exercise. Skeletal muscle usually exerts
maximal force when activated at its resting length (7). At
greater lengths, the number of potential actin/myosin (3 80
cross-bridges decreases; at shorter lengths, steric hin- E
W
drance of the contractile proteins progressively inter- ‘b 60
feres with continued shortening. IMP is theoretically de-
pendent on tension of muscle fibers at the site of mea-
surement (19) and, therefore, should be an accurate
indicator of force. EMG, however, depends on the size
and number of depolarized motor units, which can re-
main constant with changes in contracting muscle length
and, therefore, tension. 20 40 60 80 100
In caseswhere the full range of joint movement is used, torque (% max)
fascia around the relaxed skeletal muscle creates tension
FIG. 5. Tibialis anterior IMP (A; n = 9) and EMG (B; n = 8) during
(and increases IMP) as the muscle is stretched from rest-
concentric exercise. Each line represents 1 MVC from 1 subj up to
ing to maximal length (6), as occurs in an eccentric con- maximum torque. Linear regression analysis of each line yielded an R2
traction. This passive tension contributes to total muscle .
value, and mean t SE of all R" values is shown.
Downloaded from journals.physiology.org/journal/jappl (080.030.208.074) on August 12, 2020.
IMP AND EMG DURING ISOKINETIC EXERCISE 2639
300 300
A mean R* A
= 0.91 + 0.02 mean R* = 0.94 t 0.02
=200
=r:
E
E
$00
-
100
T
i+
c3 80 ; 80
z E
w 60 W
war 60
N B
N
"m 40 ; 40
E _E
5 20 'j 20
c s
20 40 60 80 100 OF * ' - ' - ' - ' - ' '
0 20 40 60 80 100
torque (% max) torque (% max)
FIG. 6. Soleus IMP (A; n = 9) and EMG (B; n = 9) during eccentric FIG. 7. Tibialis anterior IMP (A; n. = 9) and EMG (B; n = 8) during
exercise. Each line represents 1 MVC from 1 subj up to maximum eccentric exercise. Each line represents 1 MVC from 1 subj upto maxi-
torque. Linear regression analysis of each line yielded an R2 value, and mum torque. Linear regression analysi .s of each line yielded an R2
mean + SE of all R2 values is shown. value, and mean & SE of all R2 values is shown.
cause local fatigue, local EMG activity may be altered
when the total force output is unchanged. Therefore sur- measu rements must be taken at the same position and
face electrodes were used in an effort to obtain a better depth in a particula r muscle. IMP catheterization is a
overall representation of soleus and tibialis anterior myo- relatively simple and atraumatic procedure. When per-
electric activity. A common concern when using surface formed under sterile conditions by trained personnel,
electrodes is interference from stray signals of surround- risk of complications is minimal. Repeated catheteriza-
ing muscles. The tibialis anterior and soleus, however, tions required for long-term assessment, however, may
are quite accessible with surface electrodes, and cross increase the risk of tissue edema, damage, and scarring.
talk from other muscles was assumed to be minimal. Advanced technol .ogy for pressure measurements may
Although care was taken to isolate force production of minimize this risk with the development of smaller elec-
the muscles under study, the total torque measured by trical and fiber-optic transducer-tipped catheters.
the dynamometer was probably affected both agonisti- Although it is more invasive than surface EMG, IMP
tally and antagonistically by other muscles in the region. provides a more linear and direct index of individual
The exact net effect of surrounding muscles on total muscle contraction force than EMG during both concen-
force output in this study is unknown but is presumed to tric and eccentric exercise. Furthermore, exercise-in-
be slight because of precautions taken to help isolate the duced elevation of IMP is known to influence both local
muscles under study. For example, the knee joint was and systemic hemodynamics. Therefore, IMP may be
positioned at 90° flexion to avoid gastrocnemius contri- physiologically more important than EMG in studies
bution to soleus contractions. Nevertheless, this problem with cardiovascular considerations. Future studies
of agonistic and antagonistic muscle force contributions should assess the effect of muscle contraction velocity on
helps illustrate the need for a reliable, reproducible IMP-force relationships during concentric and eccentric
method of monitoring contraction force of specific mus- exercise.
cles in vivo.
Although the IMP and force correlations were nearly We gratefully acknowledge Gita Murthy, Dr. Yasuaki Kawai, and
linear in each of the nine subjects, the slopes of these Dr. Greg Breit for helpful discussions; Karen Hutchinson for technical
relationships varied greatly between subjects. This is a support; our subjects for interest and participation; and Drs. Richard
Lieber, Robert Whalen, Malcolm Cohen, and Charles Wade for review
result of intersubject variability as well as slightly vari- of the manuscript.
able catheter insertion site and depth (19). For repeat- This research was supported by National Aeronautics and Space
able assessment of muscle strength and function, IMP Administration Grants 199-14-12-04 and 199-26-12-38.
Downloaded from journals.physiology.org/journal/jappl (080.030.208.074) on August 12, 2020.
2640 IMP AND EMG DURING ISOKINETIC EXERCISE
Address for reprint requests: R. E. Ballard, Life Science Div. (239- graphic measurements with inserted wire electrodes and surface
11), NASA Ames Research Center, Moffett Field, CA 94035-1000. electrodes. Electromyogr. Clin. Neurophysiol. 10: 357-367, 1970.
13. K~RNER, L., P. PARKER, C. ALSTROM, G. B. J. ANDERSSON, P.
Received 10 August 1992; accepted in final form 15 December 1992.
HERBERTS, R. KADEFORS, G. PALMERUD, AND C. ZETTERBERG.
Relation of intramuscular pressure to the force output and myoelec-
REFERENCES tric signal of skeletal muscle. J. Orthop. Res. 2: 289-296, 1984.
1. ALEXANDER, M. J. L. Peak torque values for antagonistic muscle 14. LAWRENCE, J. H., AND C. J. DE LUCA. Myoelectric signal versus
groups and concentric and eccentric contraction types for elite force relationship in different human muscles. J. Appl. Physiol. 54:
sprinters. Arch. Phys. Med. RehabiZ. 71: 334-339, 1990. 1653-1659,1983.
2. ARMSTRONG, R. B. Mechanisms of exercise-induced delayed onset 15. MORITANI, T., M. MURO, AND A. NAGATA. Intramuscular and sur-
muscular soreness: a brief review. Med. Sci. Sports Exercise 16: face electromyogram changes during muscle fatigue. J. Appl. Phys-
529~538,1984. iol. 60: 1179-1185, 1986.
3. ASMUSSEN, E. Positive and negative muscular work. Acta Physiol. 16. PARKER, P., L. K~RNER, AND R. KADEFORS. Estimation of muscle
Stand. 28: 364-382,1952. force from intramuscular total pressure. Med. Biol. Eng. Comput.
4. BIGLAND-RITCHIE, B., AND J. WOODS. Integrated electromyogram 22:453-457,1984.
and oxygen uptake during positive and negative work. J. Physiol. 17. PERRY, J., AND G. A. BECKEY. EMG-force relationships in skeletal
Lond. 260: 267-277,1976. muscle. In: CRC Critical Reviews in Biomedical Engineering. Bos-
5. EDMAN, K. A. P., G. ELZINGA, AND M. I. M. NOBLE. Enhancement ton, MA: CRC, 1981, p. l-22.
of mechanical performance by stretch during tetanic contractions 18. SADAMOTO, T., F. BONDE-PETERSEN, AND Y. SUZUKI. Skeletal
of vertebrate skeletal muscle fibers. J. Physiol. Lond. 281: 139-155, muscle tension, flow, pressure, and EMG during sustained isomet-
1978. ric contractions in humans. Eur. J. Appl. Physiol. Occup. Physiol.
6. GERSHIJNI, D. H., N. C. YARU, A. R. HARGENS, R. L. LIEBER, R. C. 51: 395-408,1983.
O'HARA, AND W. H. AKESON. Ankle and knee position as a factor 19. SEJERSTED, 0. M., A. R. HARGENS, K. R. KARDEL, P. BLOM, 0.
modifying intracompartmental pressure in the human leg. J. Bone JENSEN, AND L. HERMANSEN. Intramuscular fluid pressure during
Jt. Surg. 66: 1415-1420,1984. isometric contraction of human skeletal muscle. J. Appl. Physiol.
7. GUYTON, A. Basic Human Physiology: Normal Function and Mecha- 56: 287-295,1984.
nisms of Disease. Philadelphia, PA: Saunders, 1977, p. 120-133. 20. SOLOMONOW, M., R. BARATTA, B. H. ZHOU, H. SHOJI, AND R.
8. KADABA, M. P., M. E. WOOTTEN, J. GAINEY, AND G. V. COCHRAN. D’AMBROSIA. Historical update and new developments on the
Repeatability of phasic muscle activity: performance of surface and EMG-force relationships of skeletal muscles. Orthopaedics 9: 1541-
intramuscular wire electrodes in gait analysis. J. Orthop. Res. 3: 1543,1986.
350-359,1985. 21. STYF, J. R., AND L. M. K~RNER. Microcapillary infusion technique
9. KAHAN, N., M. ARATOW, D. E. WATENPAUGH, R. E. BALLARD, J. for measurement of intramuscular pressure during exercise. Clin.
STYF, A. CRENSHAW, AND A. R. HARGENS. Surface EMG versus Orthop. 207: 253-262, 1986.
intramuscular EMG: correlations with joint torque during isomet- 22. TESCH, P. A., G. A. DUDLEY, M. R. DUVOISIN, B. M. HATHER, AND
ric, concentric, and eccentric exercise (Abstract). FASEB J. 6: R. T. HARRIS. Force and EMG signal patterns during repeated
A1237,1992. bouts of concentric and eccentric muscle actions. Acta Physiol.
10. KOMI, P. V. Relationship between muscle tension, EMG, and veloc- Stand. 138: 263-271,199O.
ity of contraction under concentric and eccentric work. In: New 23. THORNTON, W. E., AND J. A. RUMMEL. Muscular deconditioning
Developments in Electromyography and Clinical Neurophysiology, and its prevention in space flight. In: Biomedical Results From Sky-
edited by J. E. Desmedt. Basel: Karger, 1973, vol. 1, p. 596-606. lab, edited by R. S. Johnston and L. F. Dietlein. Washington, DC:
11. KOMI, P. V. Relevance of in vivo force measurements to human National Aeronautics and Space Administration, 1977, p. 191-197.
biomechanics. J. Biomech. 23: 23-34, 1990. 24. ZAR, J. H. Biostatistical Analysis (2nd ed.). Englewood Cliffs, NJ:
12. KOMI, P. V., AND E. R. BUSKIRK. Reproducibility of electromyo- Prentice-Hall, 1984, p. 239-241.
Downloaded from journals.physiology.org/journal/jappl (080.030.208.074) on August 12, 2020.
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (589)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5806)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- A Chair For Yoga - A Complete Gu - Eyal ShifroniDocument433 pagesA Chair For Yoga - A Complete Gu - Eyal ShifroniSudhanshu Goswami86% (28)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The 4 Hour Body Reversing Injuries Egoscue Method Cheat SheetDocument4 pagesThe 4 Hour Body Reversing Injuries Egoscue Method Cheat Sheetarun_chheda86% (7)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Thoracic Outlet SyndromeDocument678 pagesThoracic Outlet Syndromejorgeroca2009No ratings yet
- History of OrthopaedicsDocument43 pagesHistory of Orthopaedicsapi-3716867100% (4)
- TLAC 3.0 - Movement HandbookDocument16 pagesTLAC 3.0 - Movement HandbookFook Ngo100% (5)
- Des Moines Landmark ReviewDocument147 pagesDes Moines Landmark ReviewD.j. HaramieNo ratings yet
- Artigo - VibratoDocument5 pagesArtigo - VibratopedroNo ratings yet
- Abdomen CavityDocument55 pagesAbdomen CavityAjeng Fikih67% (3)
- USGDocument337 pagesUSGambar mustika100% (2)
- Post Natal Exercise PlanDocument18 pagesPost Natal Exercise PlanSathya BharathiNo ratings yet
- Full Planche Program - ZERO GRAVITYDocument10 pagesFull Planche Program - ZERO GRAVITYNhật Phú0% (1)
- BOWFLEX Manual PowerproDocument80 pagesBOWFLEX Manual Powerproapi-3830008100% (3)
- Overcoming Gravity 2nd Edition Exercise Charts - Overcoming Gravity 2nd Edition Progression ChartsDocument1 pageOvercoming Gravity 2nd Edition Exercise Charts - Overcoming Gravity 2nd Edition Progression Chartsmohihek698No ratings yet
- Ferrari M Et Al 2011Document14 pagesFerrari M Et Al 2011blaiNo ratings yet
- Sport, Health and Medical Sciences: Recent The Use of Muscle Near-Infrared Spectroscopy inDocument15 pagesSport, Health and Medical Sciences: Recent The Use of Muscle Near-Infrared Spectroscopy inblaiNo ratings yet
- Muscle Oximetry in Sports Science: A Systematic Review: Key PointsDocument20 pagesMuscle Oximetry in Sports Science: A Systematic Review: Key PointsblaiNo ratings yet
- Ates F Et Al 2019Document10 pagesAtes F Et Al 2019blaiNo ratings yet
- 01 5 CF BFDocument357 pages01 5 CF BFblaiNo ratings yet
- Fracture Classification CondensedDocument4 pagesFracture Classification Condensedaf0313No ratings yet
- Blount Disease ManagementDocument8 pagesBlount Disease ManagementRizka Rinintia SariNo ratings yet
- CSP Shoulder GuidelinesDocument101 pagesCSP Shoulder GuidelinesWilliams Ariel Chandia PérezNo ratings yet
- Pinky - Plantar FasciitisDocument34 pagesPinky - Plantar FasciitisRavindra choudharyNo ratings yet
- Group 2 TleDocument40 pagesGroup 2 Tleralph divinaNo ratings yet
- Physical-Fitness Final Rep.Document7 pagesPhysical-Fitness Final Rep.caicaiiNo ratings yet
- CH-7 Class 12Document54 pagesCH-7 Class 12Mahima FamousNo ratings yet
- Science (Skeletal and Muscular System) Grade 5Document2 pagesScience (Skeletal and Muscular System) Grade 5Nurwulan Yuni HapsariNo ratings yet
- Pert 5 - Soft Tissue Damage and HealingDocument41 pagesPert 5 - Soft Tissue Damage and HealingAlfiya HasnaNo ratings yet
- Week 2 SHS Pe-4Document31 pagesWeek 2 SHS Pe-4jasonsumaoy2006No ratings yet
- BRIEF 3.0 PDF Fill-In PDFDocument1 pageBRIEF 3.0 PDF Fill-In PDFSMIT SHARMANo ratings yet
- Fibrous DysplasiaDocument29 pagesFibrous Dysplasiadr_adjie100% (2)
- Movement - Group 2 - BiologyDocument31 pagesMovement - Group 2 - BiologyEjaz YusuffNo ratings yet
- Lab Manual - Appendicular - Skeleton - AtlasDocument58 pagesLab Manual - Appendicular - Skeleton - AtlasShao KahnNo ratings yet
- Biomechanics of The Human SpineDocument5 pagesBiomechanics of The Human SpineLusian VissNo ratings yet
- 1 s2.0 S1529943016309226 MainDocument6 pages1 s2.0 S1529943016309226 Mainhakan sonsuzlukluNo ratings yet
- Posselt DiagramDocument2 pagesPosselt DiagramScribdTranslationsNo ratings yet