Professional Documents
Culture Documents
Aha
Uploaded by
Dyako D TaherCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Aha
Uploaded by
Dyako D TaherCopyright:
Available Formats
DISCUSSION
NaOH + Et(Ac) → Na(Ac) + EtOH
The experiment was carried out by using special hydroxide and ethyl acetate. Inside the reactor, the
saponification of sodium hydroxide and ethyl acetate producing sodium acetate and ethanol. Order of
the reaction is based on the powers of the concentration which are raised in the kinetic law. Based on
result and the sample of calculation, the value of data was fitted to second order reaction.[5] From
Arrhenius’s equation, k = Ae-E/RT it show that the temperature has an effect to the reaction rate
constant. It states that when the rate constant doubles, so wills the rate of reaction. The higher the
temperature the faster the molecules move producing much more kinetic energy than normal. More
collision is happen in order for a reaction to occur and thus larger fraction of molecules to provide the
activation energy needed for the reaction. Activation energy, Ea is the minimum energy needed for the
reaction to occur.[5] Therefore, the rate law for this experiment is:
The time taken for each sample taken is from the first minute the time started and followed by the next
5th minute, 10th minute, 15th minute, 20th minute, and 25th minute. The volume of titrating sodium
hydroxide to calculate the amount of quenching hydrochloric acid, phenolphthalein is used to be
indicator of the mixture to be in neutral condition. Volume of quenching hydrochloric acid unreacted
with sodium hydroxide in sample is calculated using the amount of sodium hydroxide titrated with the
mixture. The slopes of the graph are representing the specific reaction rate constant, K. K constant can
be obtained by considering all the data obtained throughout the experiment. Based on the calculation, K
can be calculated. Since the reaction is second order, the reaction rate by the rate law is in the form of
-ra =kCACB. Then for this experiment, the volume of quenching HCL unreacted with NaOH ins sample
(ml) is 6.28 ml, 8.48 ml, 9.20 ml, 9.48 ml, 9.60 ml and 10.00 ml. Next, the volume of HCL reacted with
NaOH in sample (ml) is 3.72 ml, 1.52 ml, 0.80 ml, 0.52 ml, 0.40 ml and 0.00 ml respectively. The volume
of titrating NaOH for experiment A was increased from 15.7 ml, 21.2 ml, 23.0 ml, 23.7 ml, 24.0 ml and
25.0 ml. That means the higher the concentration of HCL, the more volume of NaOH is needed to
neutralize the mixture. After calculating all the data obtained, values of constant k can be known. From
the graph, the value k is 25.304 Lmol-1min-1
You might also like
- Batch CSTR ExperimentDocument5 pagesBatch CSTR ExperimentDyako D TaherNo ratings yet
- Chme 401 Chemical Engineering Laboratory Ii Experiment 401-4 Chemical Reactors ObjectiveDocument4 pagesChme 401 Chemical Engineering Laboratory Ii Experiment 401-4 Chemical Reactors ObjectiveDyako D TaherNo ratings yet
- Alternate Operating Methods For Improving The Performance of Continuous Stirred Tank ReactorsDocument33 pagesAlternate Operating Methods For Improving The Performance of Continuous Stirred Tank ReactorsDyako D TaherNo ratings yet
- FigureDocument1 pageFigureDyako D TaherNo ratings yet
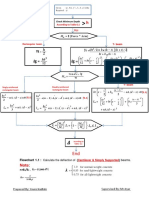
- Flowchart For Design Reinfoced Concrete 2017 PDFDocument7 pagesFlowchart For Design Reinfoced Concrete 2017 PDFDyako D TaherNo ratings yet
- Subject: Material Science & MetallurgyDocument26 pagesSubject: Material Science & MetallurgyDyako D TaherNo ratings yet
- Flowchart (Deflection) PDFDocument4 pagesFlowchart (Deflection) PDFDyako D TaherNo ratings yet
- Dyako Dyar Tahir: Experince and andDocument2 pagesDyako Dyar Tahir: Experince and andDyako D TaherNo ratings yet
- CIVIL ENGINEER DYAKO Diyar 1995 PDFDocument2 pagesCIVIL ENGINEER DYAKO Diyar 1995 PDFDyako D TaherNo ratings yet
- Introduction To Materials Science and Engineering: Mr. Pem PhakvisethDocument38 pagesIntroduction To Materials Science and Engineering: Mr. Pem PhakvisethDyako D TaherNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)