Professional Documents
Culture Documents
Ionic Compounds Worksheet-Iii
Uploaded by
tyler0 ratings0% found this document useful (0 votes)
52 views1 pageOriginal Title
IONIC COMPOUNDS WORKSHEET-III
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
52 views1 pageIonic Compounds Worksheet-Iii
Uploaded by
tylerCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
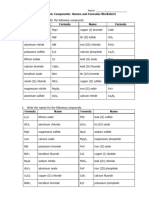
Meet Polly Atomic!
Name the following ionic compounds:
NaBr sodium bromide CaCO3 calcium carbonate
SnCl2 tin (II) chloride (NH4)2CO3 ammonium carbonate
KClO3 potassium chlorate AlPO4 aluminum phosphate
NH4F ammonium fluoride Li2SO4 lithium sulphate
CuO copper (II) oxide Cu3P2 copper (II) phosphide
Cu3PO4 copper (I) phosphate Ga2(SO4)3 gallium sulphate
FrClO3 francium chlorate Pb3N2 lead (II) nitride
SnS2 tin (IV) sulphide (NH4)2O ammonium oxide
KHCO3 potassium bicarbonate CdSO4 cadmium sulphate
CuCO3 copper (II) nitrate Fe(HCO3)2 iron (II) bicarbonate
NH4Br ammoninum bromide Cu(NO3)2 copper (II) nitrate
Write the formulas for the following ionic compounds:
lithium chlorate LiClO3 lead (II) sulfide PbS
iron (II) phosphate Fe3(PO4)2 calcium chlorate Ca(ClO3)2
sodium bicarbonate NaHCO3 sodium hydride NaH
beryllium hydroxide Be(OH)2 zinc carbonate BeCO3
copper (II) chlorate Cu(ClO3)2 ammonium sulfate (NH4)2SO4
calcium oxide CaO cesium sulfide Cs2S
barium nitrate Ba(NO3)2 lead (IV) sulfate Pb(SO4)2
potassium hydroxide KOH cesium sulfate Cs2SO4
ammonium chloride NH4Cl gallium chlorate Ga(ClO3)3
aluminum bicarbonate Al(HCO3)3 ammonium hydroxide NH4OH
You might also like
- IUPAC Name: Give The Chemical Formula For Each of The FollowingDocument1 pageIUPAC Name: Give The Chemical Formula For Each of The FollowingKiki ShofiaNo ratings yet
- Chapter - 7 Correction Naming CompoundsDocument2 pagesChapter - 7 Correction Naming CompoundsMurad IsayevNo ratings yet
- Inorganic Compounds: Chemical Name Chemical FormulaDocument6 pagesInorganic Compounds: Chemical Name Chemical FormulaFrendick LegaspiNo ratings yet
- Nomenclature Assignment Part 1Document4 pagesNomenclature Assignment Part 1marNo ratings yet
- Post 5.9. Ionic Compounds Practice - ANSWERSDocument3 pagesPost 5.9. Ionic Compounds Practice - ANSWERSAlan MartínNo ratings yet
- Chemical Formula Writing WorksheetDocument5 pagesChemical Formula Writing WorksheetÂziz ShuvoNo ratings yet
- Review #3 NomenclatureDocument1 pageReview #3 NomenclatureCassandra MachadoNo ratings yet
- Nomenclature WorksheetDocument5 pagesNomenclature WorksheetJapphetNo ratings yet
- Chemical Formula Writing Worksheet SolutionsDocument3 pagesChemical Formula Writing Worksheet SolutionsReid Pineda Creencia100% (1)
- Activity5 ChemicalformulasDocument2 pagesActivity5 ChemicalformulasJohn Hayden Dela CruzNo ratings yet
- SCH3U0 Nomenclature PracticeDocument7 pagesSCH3U0 Nomenclature PracticeArmann JohalNo ratings yet
- Baso Agno H S Cao H Co MG (Po) K Cro NaiDocument2 pagesBaso Agno H S Cao H Co MG (Po) K Cro NaiAmy BalicagNo ratings yet
- Jordan Paddock - Writing Polyatomic Names and Formulas WorksheetDocument1 pageJordan Paddock - Writing Polyatomic Names and Formulas Worksheetapi-675312232No ratings yet
- Dustin Kern - Writing Polyatomic Names and Formulas WorksheetDocument2 pagesDustin Kern - Writing Polyatomic Names and Formulas Worksheetapi-644456294No ratings yet
- Solubility ListDocument6 pagesSolubility ListpomegranatesoupNo ratings yet
- Chemistry Notes (14 - 4 - 23)Document4 pagesChemistry Notes (14 - 4 - 23)Sanchay KumarNo ratings yet
- Nomenclature Homework 1Document5 pagesNomenclature Homework 1James PerriamNo ratings yet
- What Is The Systematic Name of The Following Compound (Solved)Document7 pagesWhat Is The Systematic Name of The Following Compound (Solved)Debayanbasu.juNo ratings yet
- Naming Compounds Formulas For The Following Ionic CompoundsDocument1 pageNaming Compounds Formulas For The Following Ionic CompoundsJulie Trajano CortezNo ratings yet
- Chemical Formula Writing Exercise - Cations and AnionsDocument5 pagesChemical Formula Writing Exercise - Cations and AnionsQusai Saify100% (3)
- Chemical Formulas List For Class 10Document5 pagesChemical Formulas List For Class 10Akshita Kamboj91% (35)
- Here are the answers to the Counting Atoms worksheet:1) CaF22) Be(OH)2 3) NO24) Al2(SO4)35) NH4NO36) S2F2 7) Na2CO38) CH49) PCl310) Mg(OH)211) K3PO4 12) P2Br413) NH3Document14 pagesHere are the answers to the Counting Atoms worksheet:1) CaF22) Be(OH)2 3) NO24) Al2(SO4)35) NH4NO36) S2F2 7) Na2CO38) CH49) PCl310) Mg(OH)211) K3PO4 12) P2Br413) NH3Supremo DelagerNo ratings yet
- San Pedro College Senior High Chemistry Compounds ListDocument9 pagesSan Pedro College Senior High Chemistry Compounds ListEvann Myelle MontejoNo ratings yet
- Chemical FormulaeDocument3 pagesChemical Formulaeparth chakre100% (2)
- Naming Ionic Compounds Practice Worksheet - SolutionsDocument3 pagesNaming Ionic Compounds Practice Worksheet - SolutionsJa Son Tonogbanua100% (1)
- 2 WeekDocument2 pages2 WeekAna Carballo TrabazoNo ratings yet
- 1.5B Solutions For Ionic Compounds, Extra ExercisesDocument1 page1.5B Solutions For Ionic Compounds, Extra ExercisesDaniel StandringNo ratings yet
- Chemical Substance Formula: List of Chemical Substances With FormulaeDocument3 pagesChemical Substance Formula: List of Chemical Substances With FormulaeJaithri Mualakala100% (1)
- Chemical FormulaDocument2 pagesChemical FormulaCessnirpLyma RaguroNo ratings yet
- 2.4.3 Chemical Formula and Naming Practice QuestionsDocument7 pages2.4.3 Chemical Formula and Naming Practice Questionsphat.vuongNo ratings yet
- WS#2 Naming and Writing Inorganic CompoundsDocument6 pagesWS#2 Naming and Writing Inorganic CompoundsOw ZeeNo ratings yet
- Laboratory Inventory 1 Chemistry Lab ChemicalsDocument4 pagesLaboratory Inventory 1 Chemistry Lab Chemicalspdqcmusa54No ratings yet
- Ionic Compounds Names and Formulas Worksheet AnswersDocument2 pagesIonic Compounds Names and Formulas Worksheet AnswersShayan UzzamanNo ratings yet
- Naming Inorganic Compound Practice SheetDocument4 pagesNaming Inorganic Compound Practice SheetWichel AnnNo ratings yet
- Max Hyman - Writing Mixed Names and Formulas WorksheetDocument1 pageMax Hyman - Writing Mixed Names and Formulas Worksheetapi-673504994No ratings yet
- Chemical Compounds List and FormulasDocument8 pagesChemical Compounds List and FormulasMOhd HisyamNo ratings yet
- Mixed FormulasDocument3 pagesMixed FormulasasierNo ratings yet
- Daftar Nama Kimia Serta Rumus KimianyaDocument7 pagesDaftar Nama Kimia Serta Rumus KimianyaDewi KuperNo ratings yet
- Chemical Formula Binary Ionic CompoundsDocument2 pagesChemical Formula Binary Ionic CompoundsRamisNo ratings yet
- Chemical Formulas and NamesDocument8 pagesChemical Formulas and Namesalbenis_batistaNo ratings yet
- Symbol - Equations - Homework RMDocument2 pagesSymbol - Equations - Homework RMayaanrayhaanNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetOlivia DitzelNo ratings yet
- Ionic Compounds WorksheetDocument2 pagesIonic Compounds WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSam JoNo ratings yet
- Review 1 WorksheetDocument2 pagesReview 1 WorksheetsupremeNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetBrillantes JYNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheet김동주No ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice WorksheetSunshine LadyNo ratings yet
- Naming Ionic Compounds Practice WorksheetDocument2 pagesNaming Ionic Compounds Practice Worksheetarlene serdiniaNo ratings yet
- Common Chemical FormulasDocument3 pagesCommon Chemical FormulasFfrenchNo ratings yet
- Nomenclature Worksheet NDocument2 pagesNomenclature Worksheet NVictor GarciaNo ratings yet
- More Extra Nomenclature Practice - KEYDocument10 pagesMore Extra Nomenclature Practice - KEYelitzelmartinez21No ratings yet
- Eleazar - Quiz#3Document2 pagesEleazar - Quiz#3ゆかりNo ratings yet
- Nomenclature WorksheetDocument2 pagesNomenclature WorksheetJoseph GagnonNo ratings yet
- Give Correct Formulas For These Type I Binary CompoundsDocument5 pagesGive Correct Formulas For These Type I Binary CompoundsJeanette HernandezNo ratings yet
- Tugas Prak - Kimia Analitik - 1DDocument3 pagesTugas Prak - Kimia Analitik - 1Dmuhammadsalmanfath05No ratings yet
- Microsoft Word - Ionic-CovalentNameRace1Document2 pagesMicrosoft Word - Ionic-CovalentNameRace1cen BsitNo ratings yet
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Gravimetric Analysis: International Series of Monographs in Analytical Chemistry, Vol. 7From EverandGravimetric Analysis: International Series of Monographs in Analytical Chemistry, Vol. 7No ratings yet
- Community Information Bulletin Mandatory Masks and Staggered Entry August 26 2020Document6 pagesCommunity Information Bulletin Mandatory Masks and Staggered Entry August 26 2020tylerNo ratings yet
- Community Information Bulletin Mandatory Masks and Staggered Entry August 26 2020Document6 pagesCommunity Information Bulletin Mandatory Masks and Staggered Entry August 26 2020tylerNo ratings yet
- Community Information Bulletin Mandatory Masks and Staggered Entry August 26 2020Document6 pagesCommunity Information Bulletin Mandatory Masks and Staggered Entry August 26 2020tylerNo ratings yet
- Community Information Bulletin Mandatory Masks and Staggered Entry August 26 2020Document6 pagesCommunity Information Bulletin Mandatory Masks and Staggered Entry August 26 2020tylerNo ratings yet
- Return To School Student Health and Well-Being Session For Parent With Dr. Monica Hau September 3Document1 pageReturn To School Student Health and Well-Being Session For Parent With Dr. Monica Hau September 3tylerNo ratings yet
- Community Information Bulletin Secondary School Re-Entry Update August 31 2020Document2 pagesCommunity Information Bulletin Secondary School Re-Entry Update August 31 2020tylerNo ratings yet