Professional Documents
Culture Documents
EIA M1-Ktunotes - in
EIA M1-Ktunotes - in
Uploaded by
Jenny Guarda JudanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
EIA M1-Ktunotes - in
EIA M1-Ktunotes - in
Uploaded by
Jenny Guarda JudanCopyright:
Available Formats
MODULE 1
POLLUTION
Pollution is the effect of undesirable changes in our surroundings that have harmful effects on plants,
animals and human beings. Pollution is thus direct or indirect change in any component of the biosphere
that is harmful to the living components and in particular undesirable for man, affecting adversely the
industrial progress, cultural and natural assets or general environment of living society.
CLASSIFICATION OF POLLUTION
Types of pollutions are classified in different ways. On the basis of the type of environmental being
polluted, we may recogniseAir Pollution, Water Pollution, and Land (Soil) Pollution etc. On the basis
of the kind of pollutant involved, we mayhave sulphur dioxide pollution, fluoride pollution, carbon
monoxide pollution, smoke pollution, lead pollution, mercury pollution, solid waste pollution, radioactive
pollution, noise pollution etc.
1. Air Pollution
Air pollution is contamination of the indoor or outdoor environment by any chemical, physical or
biological agent that modifies the natural characteristics of the atmosphere.
Sources of Air Pollution:
• Household combustion devices.
• Motor vehicles.
• Industrial facilities.
• Forest fires
Prevention of Air Pollution:
• The coal fuel should be replaced with gas fuel to control the air pollution.
• The automobiles must be designed with emission control system.
• The wastes must be removed and recycled in the industrial plants and refineries.
• Plants like pine and ribes need to be planted to metabolize the nitrogen oxides and other pollutants.
• Timely servicing of the vehicles helps to keep it in a good condition, and also minimizes fuel
exhaustion
• Using public transportation helps to prevent the air pollution
• Using alternative energy sources like solar energy, hydroelectric energy, and wind energy
1
Downloaded From www.ktunotes.in
2. Water Pollution
Water pollution is the contamination of water bodies (e.g. lakes, rivers, oceans, aquifers and
groundwater). Water pollution occurs when pollutants are discharged directly or indirectly into water
bodies without adequate treatment to remove harmful compounds. Water pollution affects plants and
organisms living in these bodies of water. In almost all cases the effect is damaging not only to individual
species and populations, but also to the natural biological communities.
Water Pollutants:-
• Plastics
• Pesticides
• Heavy metals
• Sewage
• Radioactive waste
• Thermal effluents
• Detergents
• Chloroform
• Food processing waste (fats and grease)
• Insecticides and herbicides.
• Petroleum hydrocarbons, (gasoline, diesel fuel, jet fuels, and fuel oil).
• Lubricants (motor oil).
Prevention of Water Pollution:
• Conserve water
• Use environment friendly household products, such as washing powder, household cleaning agents etc.
• Take great care not to overuse pesticides and fertilizers.
• Don’t throw litter into rivers, lakes or oceans. Help clean up any litter you see on beaches or in rivers
and lakes, make sure it is safe to collect the litter and put it in a nearby dustbin.
3. Soil Pollution
Soil contamination or soil pollution is caused by the presence of xenobiotic (human-made) chemicals or
other alteration in the natural soil environment. It is typically caused by
• Industrial activity.
• Agricultural chemicals.
• Improper disposal of waste.
Prevention of Soil Pollution:
• Limit the use of fertilizers and pesticides
• Awareness about biological control methods and their implementation
• The grazing must be controlled and forest management should be done properly
• The afforestation and reforestation must take place
• Proper preventive methods like shields should be used in areas of wind erosion and wind breaks
4. Noise Pollution
Noise pollution is displeasing or excessive noise that may disrupt the activity or balance of human or
animal life. Noise means disgust or discomfort hearing from environment.
Sources of Noise Pollution:
• Machines.
• Transportation systems.
• Motor vehicles.
• Aircrafts.
• Trains.
• Poor urban planning.
Downloaded From www.ktunotes.in
5. Radioactive Pollution
It occurs when radioactive metals disintegrate releasing dangerous beta rays which can cause cancer and
other mutative diseases. This type of pollution can occurs by the dumping of radioactive waste from
nuclear power plants into water bodies and soil.
POLLUTANTS OF ENVIRONMENT
Any substance which causes pollution is called a pollutant. A Pollutant may thus include any chemical or
geochemical (dust, sediment, grit etc.) substance, biotic component or its product, or physical factor
(heat) that is released intentionally by man into the environment in such a concentration that may have
adverse harmful or unpleasant effects.
A Pollutant has also been defined as “any solid, liquid or gaseous substance present in such concentration
as may be or tend to be injurious to the environment.” Pollutants are the residues of things we use and
throw away. There are many sources of such pollutants. The lakes and rivers are polluted by water from
chemical and other factories, and the air by gases of automobile exhausts, industries, thermal power
plants etc.
Types of Pollutants:
The various principal pollutants which pollute air, water and land are as follows:
(1) Deposited matter. Smoke, tar, dust, etc.
(2) Gases. Oxides of nitrogen (NO, NO2), sulphur (SO,), carbon monoxide, halogens, (chlorine, bromine,
iodine)
(3) Acids droplets. Sulphuric acid, nitric acid etc.
(4) Fluorides
(5) Metals. Mercury, lead, iron, zinc, nickel, tin, cadmium, chromium etc.
(6) Agrochemicals. Biocides (like pesticides, herbicides, fungicides, nematicides, bactericides,
weedicides etc. and fertilisers.
(7) Complex organic oxidants. Benzene, ether, acetic acid, benzpyrenes etc.
(8) Photochemical oxidants. Photochemical smog, ozone, Peroxyacetyl Nitrate (PAN), Peroxy Benzoyl
Nitrate, nitrogen oxides, aldehydes, ethylene etc.
(9) Solid domestic wastes
(10) Radioactive waste
(11) Noise
A. Classification of pollutants on the basis of nature
1. Pollution caused by gaseous wastes – Eg. CO, CO2, SO2, NO2, O3, Smog gases, etc.
2. Pollution caused by liquid wastes-Eg. Acids, Alkalis, Poisonous substances, Pesticides, Herbicides,
etc.
3. Pollution caused by solid wastes- Eg.Garbage, Rubbish, Ashes, Construction wastes, Dead animals
Waste, Agricultural waste, etc.
B. Classification of pollutants on the basis of decomposition
1. Non degradable pollutants
2. Degradable pollutants
Downloaded From www.ktunotes.in
AIR POLLUTION
Air Pollution may be defined as any atmospheric condition in which certain substances are present in
such concentrations that they can produce undesirable effects on man and his environment. Air Pollutant
is any solid, liquid or gaseous substance present in the atmosphere in such concentration, as may be or
tend to be injurious to human beings or other living creatures or plants or property or environment. These
substances include gases (sulphur oxides, nitrogen oxides, carbon monoxide, hydrocarbons etc.),
particulate matter (smoke, dust, fumes, and aerosols), radioactive materials and many others. Most of
these substances are naturally present in the atmosphere in low (background) concentrations and are
usually considered to be harmless. Thus, a particular substance can be considered an air pollutant only
when its concentration is relatively high compared with the background value and causes adverse effects.
These pollutants are dispersed throughout the world's atmosphere in concentrations high enough to
gradually cause serious health problems. Serious health problems can occur quickly when air pollutants
are concentrated.
SOURCES OF AIR POLLUTION
● Stationary and Area Sources
A stationary source of air pollution refers to an emission source that does not move, also known as a
point source. Stationary sources include factories, power plants, dry cleaners and degreasing operations.
The term area source is used to describe many small sources of air pollution located together whose
individual emissions may be below thresholds of concern, but whose collective emissions can be
significant.
Residential wood burners are a good example of a small source, but when combined with many other
small sources, they can contribute to local and regional air pollution levels. Area sources can also be
thought of as non-point sources, such as construction of housing developments, dry lake beds, and
landfills.
● Mobile Sources
A mobile source of air pollution refers to a source that is capable of moving under its own power. In
general, mobile sources imply "on-road" transportation, which includes vehicles such as cars, sport utility
vehicles, and buses. In addition, there is also a "non-road" or "off-road" category that includes gas-
powered lawn tools and mowers, farm and construction equipment, recreational vehicles, boats, planes,
and trains.
● Agricultural Sources
Agricultural operations, those that raise animals and grow crops, can generate emissions of gases and
particulate matter. For example, animals confined to a barn or restricted area (rather than field grazing),
produce large amounts of manure. Manure emits various gases, particularly ammonia into the air. This
ammonia can be emitted from the animal houses, manure storage areas, or from the land after the manure
is applied. In crop production, the misapplication of fertilizers, herbicides, and pesticides can potentially
result in aerial drift of these materials and harm may be caused.
● Natural Sources
Although industrialization and the use of motor vehicles are overwhelmingly the most significant
contributors to air pollution, there are important natural sources of "pollution" as well. Wildland fires,
dust storms, and volcanic activity also contribute gases and particulates to our atmosphere
CLASSIFICATION OF AIR POLLUTANTS
The variety of matter emitted to the atmosphere by natural and anthropogenic sources is so diverse that it
is difficult to classify air pollutants. Usually, they are classified as either primary or secondary.
(i) PRIMARY POLLUTANTS
Primary pollutants are substances directly produced and emitted by a process (from source), such as ash
from a volcanic eruption or the carbon monoxide gas from a motor vehicle exhaust.
Downloaded From www.ktunotes.in
Major primary pollutants include:
● Suspended particulate matter (SPM), such as smoke, dust, fumes, mist and spray, traces of metals,
like lead, cadmium, copper, nickel, mercury and iron.
● Sulfur oxides (SOx) [SO2, SO3]
● Nitrogen oxides (NOx) [NO, NO2, N2O] Carbon monoxide (CO)
● Carbon dioxide (CO2), a greenhouse gas
● Volatile organic compounds (VOC), such as CFC
● Hydrocarbons including the photo-chemically reactive aliphatic hydrocarbons like alenes and non-
reactive alkanes, and carcinogenic substances chiefly consisting of aromatic hydrocarbons.
● Allergic agents like pollens and spores and Radioactive substances
● Certain less important primary pollutants are H2S and HF and other fluorides, methyl and ethyl
mercaptains etc., which are usually rarely found in our atmosphere, although if present, may prove
quite harmful.
(ii) SECONDARY POLLUTANTS
Secondary pollutants are not directly emitted. Rather, they form in the atmosphere by chemical
interactions among primary pollutants and normal atmospheric constituents. The primary pollutants often
react with one another or with water vapour, aided by sunlight to form secondary pollutants.
Major Secondary pollutants include:
● Sulphuric acid (H2SO4)
● Particulate matter formed from gaseous primary pollutants
● Compounds in photochemical smog, such as nitrogen dioxide Ground level ozone (O3)
● Peroxyacetyl nitrate (PAN) Formaldehydes etc.
Note that some pollutants may be both primary and secondary: that is, they are both emitted directly and
formed from other primary pollutants.
Pollutants can further be classified as criteria pollutants, hazardous air pollutants and non-criteria air
pollutants.
Criteria Air Pollutants
Air pollution contributes to a wide variety of adverse health effects. Criteria air contaminants, or criteria
pollutants, are a set of air pollutants that cause smog, acid rain, and other health hazards. Criteria
pollutants are typically emitted from many sources in industry, mining, transportation, electricity
generation and agriculture. In most cases they are the products of the combustion of fossil fuels or
industrial processes.
The six criteria air contaminants were the first set of pollutants recognized by the United States
Environmental Protection Agency as needing standards on a national level. The Clean Air Act designed
to control air pollution requires Environmental Protection Agency (EPA) to set US National Ambient Air
Quality Standards (NAAQS) for the six pollutants considered harmful to public health and the
environment. The NAAQS are health based and the EPA sets two types of standards: primary and
secondary. The primary standards are designed to protect the health of 'sensitive' populations such as
asthmatics, children, and the elderly. The secondary standards are concerned with protecting the
environment. They are designed to address visibility, damage to crops, vegetation, buildings, and
animals. Since EPA uses health-based criteria to set primary national ambient air quality standards
(NAAQS) for six of the most common air pollutants— carbon monoxide, lead, ground-level ozone,
particulate matter, nitrogen dioxide, and sulfur dioxide—leading these six pollutants to become known as
―criteria” air pollutants (or simply “criteria pollutants). In India we have our own standards and limits
for these criteria pollutants.
PHOTOCHEMICAL REACTIONS TAKING PLACE IN ATMOSPHERE
Molecules in the atmosphere are continually moving and colliding with one another, as described by the
kinetic-molecular theory. The atmosphere is also continually illuminated during daylight hours. As a
result, absorption of light energy by atmospheric molecules can cause photochemical reactions, reactions
that would not occur at normal atmospheric temperatures in the absence of light. Such reactions play an
5
Downloaded From www.ktunotes.in
important role in determining the composition of the atmosphere itself and the fate of many chemical
species that contribute to air pollution.
(i) FORMATION OF GROUND LEVEL OZONE (O 3)
NOTE:
VOC – Volatile Organic Compounds(eg. CFCs)
RO2 represents any of a number of chains of organics with an O 2 attached (replacing H in the
original chain)
M- foreg. CO + 2O2 -> CO2 + O3
(ii) FORMATION OF PEROXY ACETYL NITRATE (PAN)
In the atmosphere peroxyacyl nitrates are not generated as they are; they are generated in situ by
photochemical reactions having NOx and VOC as precursors. Depending on organic radical, peroxyacyl
nitrates can be: peroxy acetyl nitrates (PAN): CH3C(O)OONO2; peroxypropionyl nitrates (PPN):
CH3CH2C(O)OONO2; peroxy n-butyryl nitrates (PnBN): CH3CH2CH2C(O) OONO2 etc. Among these,
PAN plays an important in atmospheric chemistry. The reactions of PAN formation are based on
generation of acetyl radicals by radiation of some VOC (hydrocarbons, alcohols, aldehides).
Downloaded From www.ktunotes.in
PAN stability in the atmosphere is limited (35 minutes). The daytime evolution of oxidant smog
composition is expressed by PAN/O3 ratio. The ratio decreases with increasing temperatures. This
confirm of PAN thermal decomposition mechanism (1). This ratio has a maximum value during night
(the temperature diminution promotes PAN stability) and a minimum value at now (mid-day), when
temperature is higher. A secondary maximum is noticed in the morning when hydroxyl radicals
availability is increased (a more intensive road traffic) promoting PAN generation.
PAN impact upon human health, plants and environment in general justifies specialists’ interest for the
study of this compound. The conversion of human exposure expressed by ppb (μg/person/day). 1 ppb
PAN = 4,95μg/m3 la 25ºC and 1 atm and for the 23 m3 of air which are daily represent 114 μg/person/day
inhaled. The inhaled doze would be 570 μg/person/day if PAN concentration in inhaled air is 5 ppb.
PRIMARY AIR POLLUTANTS
● CARBON MONOXIDE (CO):
It is an odorless, colorless gas formed by incomplete combustion of carbon in fuel. The main source is
motor vehicle exhaust, along with industrial processes and biomass burning. Other major CO sources are
wood-burning stoves, incinerators and industrial sources. Carbon monoxide binds to hemoglobin in red
blood cells, reducing their ability to transport and release oxygen throughout the body. Low exposures
can aggravate cardiac ailments, while high exposures cause central nervous system impairment or death.
It also plays a role in the generation of ground-level ozone. When CO enters the bloodstream, it reduces
the delivery of oxygen to the body's organs and tissues. Health threats are most serious for those who
suffer from cardiovascular disease. Exposure to elevated CO levels can cause impairment of visual
perception, manual dexterity, learning ability and performance of complex tasks.
Carbon monoxide (CO) is highly poisonous and is generally classified as asphyxiant. The major sources
of carbon monoxide in the environment are the man-made sources arising from the incomplete burning of
carbon-based fuels including petrol, diesel, and wood. About 420 million tons of CO is discharged into
the atmosphere per year through human activity out of which 99.5% is contributed by transportation
alone. It is toxic to humans and animals when encountered in higher concentrations. CO possesses about
200 times affinity for blood haemoglobin (Hb) than oxygen. Eventually when inhaled, CO replaces O 2
from the haemoglobin, and form what is known as carboxyhaemoglobin (CO. Hb). This carboxy-
haemoglobin is of no used for respiratory purpose and when half of the haemoglobin is used up to form
carboxy-haemoglobin, death becomes certainty due to asphyxiation (lack of oxygen). Persons dying of
CO inhalation exhibit characteristic bright pink colour of flesh due to the presence of pink
colouredcarboxyhaemoglobin in blood. CO also affect the central nervous system and is responsible for
heart attacks and high mortality rates. In cities, it is found in as high concentrations as about 60 mg/m3
(54 ppm) with still higher concentration as about 60 mg/m3. The specified standard for CO under US
Ambient Air Standards is 10 mg/m3 (9ppm). The national ambient air quality standards in India prescribe
the maximum permissible concentration of CO on hourly weighted average value of 4mg/m3 (3.6 ppm)
for residential areas.
Downloaded From www.ktunotes.in
● NITROGEN OXIDES(NOx):
Nitrogen oxides include various nitrogen compounds like nitrogen dioxide (NO 2) and Nitric oxide (NO).
(NO and NO2, referred together as NOx ) are highly reactive gases formed when oxygen and nitrogen
react at high temperatures during combustion or lightning strikes. Nitrogen present in fuel can also be
emitted as NOx during combustion. These compounds play an important role in the atmospheric
reactions that create ozone (O3) and acid rain. Individually they may affect ecosystems both on land and
in water. In the atmosphere NOx reacts with volatile organic compounds (VOCs) and carbon monoxide
to produce ground-level ozone through a complicated chain reaction mechanism. Like sulfuric acid, nitric
acid contributes to acid deposition and to aerosol formation. NO forms when fuels are burned at high
temperatures. The two major emissions sources are transportation vechicles and stationary combustion
sources such as electric utility and industrial boilers. Nitrogen dioxide (NO 2) is a reddish-brown , toxic
gas with biting odour that is present in all urban atmospheres which is produced from burning of fossil
fuels . It can irritate the lungs, cause bronchitis and pneumonia and lower resistance to respiratory
infections. The major mechanism for the formation of NO2 in the atmosphere is the oxidation of nitric
oxide which is produced by most combustion processes. These are highly reactive gases formed when
oxygen and nitrogen react at high temperatures during combustion or lightning strikes.
Out of seven known varieties of oxides of nitrogen only nitric oxide (NO) and nitrogen dioxide (NO2)
are found to be somewhat injurious to human health. The sum of nitric oxide (NO) and NO2 is
commonly called nitrogen oxides or NOx. At high concentrations, these oxides are very toxic. NO2 is
considered more injurious than NO. The main source of man-made NOx forms are coal, oil, natural gas
and motor vehicle fuel combustion. However, the highest concentration of nitrogen oxides in gaseous
emissions occurs in effluents from industries where nitric acid is produced or used in chemical reaction.
Also, effluent from large power plants and exhaust from furnaces contribute to NOx. In addition to
contributing to the formation of ground-level ozone, and fine particle pollution, NO2 is linked with a
number of adverse effects on the respiratory system. It is a brown pungent gas and cause irritation even at
a concentration of 0.12ppm. Exposure to nitrogen dioxide has been associated with a variety of health
effects. Effects include respiratory symptoms, especially among asthmatic children, and respiratory-
related emergency department visits and hospital admissions, particularly for children and older adults
decrease the concentration of stratospheric ozone which prevent harmful ultraviolet radiation. The
specified maximum permissible average annual standard for NO2 for residential areas, under U.S.
Ambient Air Quality Standards is 100 μg/m3, while under Indian Ambient Air Standards its value is 60
μg/m3 i.e. 0.032 ppm.
● SULPHUR DIOXIDE (SO2):
It is a gas formed when sulphur is exposed to oxygen at high temperatures during fossil fuel combustion,
oil refining, or metal smelting. SO2 is toxic at high concentrations, but its principal air pollution effects
are associated with the formation of acid rain and aerosols. SO2 dissolves in cloud droplets and oxidizes
to form sulphuric acid (H2SO4), which can fall to Earth as acid rain or snow or form sulphate aerosol
particles in the atmosphere. Since coal and petroleum often contain sulphur compounds, their combustion
generates sulphur dioxide. Further oxidation of SO2, usually in the presence of a catalyst such as NO2,
forms H2SO4, and thus acid rain. Thus SO2 is a primary contributor to acid rain, which causes
acidification of lakes and streams and can damage trees, crops, historic buildings and statues. High
concentrations of sulphur dioxide (SO2) affect breathing and may aggravate existing respiratory and
cardiovascular disease. In addition, sulphur compounds in the air contribute to visibility impairment in
large parts of the country. This is especially noticeable in national parks. Sulphur dioxide (SO 2) is
released primarily from burning fuels that containsulphur (like coal, oil and diesel fuel). Stationary
sources such as coal- and oil-fired power plants, steel mills, refineries, pulp and paper mills, and
nonferrous smelters are the largest releasers.
The largest sources of SO2 emissions are from fossil fuel combustion at power plants (73%) and other
industrial facilities (20%). Smaller sources of SO2 emissions include industrial processes such as
extracting metal from ore, and the burning of high sulphur containing fuels by locomotives, large ships,
and non-road equipment. SO2 is a colourless gas with sharp pungent odour and it is linked with a number
of adverse effects on the respiratory system. It is an irritant gas and when inhaled, affects mucous
8
Downloaded From www.ktunotes.in
membrane. It increases breathing rate and causes oxygen deficits in the body leading to bronchial spasms
in some affected persons. Patients of asthma are very badly affected by this pollutant. Some quantity of
SO2 may oxidized in air to form sulphur trioxide SO 3, which when inhaled, may react with the body
fluids to form sulphuric acid, which is a very strong corrosive acid. SO 3 thus causes high and worst
irritation even at low concentrations, leading to severe bronchospasm ion. SO 2 is also responsible for
causing acidity in fogs, smokes and in rains, and hence the major source of corrosion of buildings and
metal objects. The maximum permissible average annual specified standard for residential areas for SO 2
under U.S. Ambient Air quality standards as well as for Indian Standards is 60 μg/m3 which
approximately 0.023 ppm at 20°C.
● VOLATILE ORGANIC COMPOUNDS (VOCS)
Volatile organic compounds (VOC) are defined as chemicals that participate in forming ozone (O3).
Ozone is a respiratory toxicant. The class of VOCs includes many specific chemicals which may also
cause adverse health effects in their own right (such as cancer or reproductive toxicity). VOCs are
emitted from diverse sources, including automobiles, chemical manufacturing facilities, drycleaners,
paint shops and other commercial and residential sources that use solvent and paint. VOC emissions form
O3 through complex chemical reactions with oxides of nitrogen (NOx) in the presence of sunlight. The
importance of VOCs as precursors depends on their chemical structure and atmospheric lifetime, which
can vary considerably from compound to compound. Large VOCs oxidize in the atmosphere to produce
nonvolatile chemicals that condense to form aerosols. Short-lived VOCs interact with NOx to produce
high ground-level ozone in polluted environments. Methane (CH4), the simplest and most long-lived
VOC, is of importance both as a greenhouse gas and as a source of background tropospheric ozone.
● PARTICULATE MATTER (PM)
Particulate matter Particulates, alternatively referred to as particulate matter (PM) or fine particles, are
tiny particles of solid or liquid suspended in a gas. In contrast, aerosol refers to particles and the gas
together. Particulate matter (PM) includes dust, dirt, soot, smoke and liquid droplets directly emitted into
the air by sources such as factories, power plants, cars, construction activity, fires and natural windblown
dust. Particles formed in the atmosphere by condensation or the transformation of emitted gases such as
SO2 and VOCs are also considered particulate matter Sources of particulate matter can be man-made or
natural. Some particulates occur naturally, originating from volcanoes, dust storms, forest and grassland
fires, living vegetation, and sea spray. Human activities, such as the burning of fossil fuels in vehicles,
power plants and various industrial processes also generate significant amounts of aerosols. Particulate
matter (PM) is a mixture of particles that can adversely affect human health, damage materials and form
atmospheric haze that degrades visibility. PM is usually divided up into different classes based on size,
ranging from total suspended matter (TSP) to PM-10 (particles less than 10 microns in aerodynamic
diameter) to PM-2.5 (particles less than 2.5 microns). In general, the smallest particles pose the highest
human health risks. PM exposure can affect breathing, aggravate existing respiratory and cardiovascular
disease, alter the body's defense systems against foreign materials, and damage lung tissue, contributing
to cancer and premature death. Individuals with chronic obstructive pulmonary or cardiovascular disease,
asthmatics, the elderly and children are most sensitive to the effects of PM.
● AMMONIA (NH3)
It is emitted from agricultural processes. Ammonia is a compound with the formula NH 3. It is normally
encountered as a gas with a characteristic pungent odor. Ammonia contributes significantly to the
nutritional needs of terrestrial organisms by serving as a precursor to foodstuffs and fertilizers.
Ammonia, either directly or indirectly, is also a building block for the synthesis of many
pharmaceuticals. Although in wide use, ammonia is both caustic and hazardous. Ammonia is a colorless,
pungent, hazardous caustic gas composed of nitrogen and hydrogen. Though ammonia is used for
different applications in many sectors, agriculture is its largest consumer and producer. Livestock
farming, animal waste and fertilizer application are the biggest sources of atmospheric ammonia
emissions within the agricultural sector. Gaseous ammonia is a dangerous air pollutant. Breathing in
large amounts can cause death.
Downloaded From www.ktunotes.in
● HYDROCARBONS (HC)
A class of burned or partially burned fuel, hydrocarbons are toxins. Hydrocarbons are a major contributor
to smog, which can be a major problem in urban areas. Prolonged exposure to hydrocarbons contributes
to asthma, liver disease, lung disease, and cancer. Regulations governing hydrocarbons vary according to
type of engine and jurisdiction; in some cases, "non-methane hydrocarbons" are regulated, while in other
cases, "total hydrocarbons" are regulated.
● LEAD(Pb)
Lead (Pb) is a widely used metal that, once released to the environment, can contaminate air, food, water,
or soil. Exposures to even small amounts of lead over a long time can accumulate to reach harmful levels.
Harmful effects may therefore develop gradually without warning. Short-term exposure to high levels of
lead may also cause harm. Lead can adversely affect the nervous, reproductive, digestive, cardiovascular
blood-forming systems, and the kidney. In men, adverse reproductive effects include infertility etc .In
women, adverse reproductive effects include reduced fertility, still-birth, or miscarriage. Children are a
sensitive population as they absorb lead more readily and their developing nervous system puts them at
increased risk for lead-related harm, including learning disabilities.
Lead gasoline additives, non-ferrous smelters, and battery plants are the most significant contributors to
Pb emissions into the atmosphere. Other stationary sources are waste incinerators, utilities,
paints and lead-acid battery manufacturers
SECONDARY POLLUTANTS
● OZONE
Ozone (O3) is a highly reactive gas composed of three oxygen atoms. It is both a natural and a man-made
product that occurs in the Earth's upper atmosphere (the stratosphere) and lower atmosphere (the
troposphere).Tropospheric ozone – what we breathe - is formed primarily from photochemical reactions
between two major classes of air pollutants, volatile organic compounds (VOC) and nitrogen oxides
(NOX).Ozone (O3) is the major component of smog. Although O3 in the upper atmosphere is beneficial
because it shields the earth from the sun's harmful ultraviolet radiation, high concentrations of O 3 at
ground level are a major health and environmental concern. The reactivity of O 3 causes health problems
because it damages lung tissue, reduces lung function and sensitizes the lungs to other irritants. Scientific
evidence indicates that ambient levels of O3 not only affect people with impaired respiratory systems,
such as asthmatics, but healthy adults and children as well. Exposure to O 3 for several hours at relatively
low concentrations has been found to significantly reduce lung function and induce respiratory
inflammation in normal, healthy people during exercise.
O3 is not usually emitted directly but is formed through complex chemical reactions in the atmosphere.
Precursor compounds like volatile organic compounds (VOC) and oxides of nitrogen (NOx) react to form
O3 in the presence of sunlight. These reactions are stimulated by ultraviolet radiation and temperature, so
peak O3 levels typically occur during the warmer times of the day and year
10
Downloaded From www.ktunotes.in
● PAN (PEROXY ACETYL NITRATE)
Smog is caused by the interaction of some hydrocarbons and oxidants under the influence of sunlight
giving rise to dangerous peroxy acetyl nitrate (PAN. It is a secondary pollutant present in photochemical
smog. It is thermally unstable and decomposes into peroxyethanoyl radicals and nitrogen dioxide gas.
Peroxyacetyl nitrate, or PAN, is an oxidant that is more stable than ozone. Hence, it is more capable of
long-range transport than ozone. It serves as a carrier for oxides of nitrogen (NOx) into rural regions and
causes ozone formation in the global troposphere.
● PHOTOCHEMICAL SMOG
Photochemical smog is a mixture of pollutants which includes particulates, nitrogen oxides, ozone,
aldehydes, peroxyethanoyl nitrate (PAN), unreacted hydrocarbons, etc. The smog often has a brown
haze due to the presence of nitrogen dioxide. It causes painful eyes. Particulate matter formed from
gaseous primary pollutants and compounds in photochemical smog .Smog is a kind of air pollution; the
word "smog" is a combination of smoke and fog. Classic smog results from large amounts of coal
burning in an area caused by a mixture of smoke and sulfur dioxide. Modern smog does not usually come
from coal but from vehicular and industrial emissions that are acted on in the atmosphere by sunlight to
form secondary pollutants that also combine with the primary emissions to form photochemical smog.
11
Downloaded From www.ktunotes.in
● AEROSOLS AND MISTS (H2SO4)
Aerosols and mists are very fine liquid droplets that cannot be effectively removed using traditional
packed scrubbers. These droplets can be formed from gas phase hydrolysis of halogenated acids (HCl,
HF, HBr), metal halides, organohalides, sulfur trioxide (SO3), and phosphorous pentoxide (P2O5). Mists
are dispersions of liquids in gases, in particular in air. They are formed during nebulization of liquids,
during condensation from the vapour phase and during chemical processes (example: oil mist, hydrogen
chloride in damp air). After sedimentation, the liquid particles of mists lose their particulate form, unlike
undissolved solid particles which generally retain their form. Aerosols are multiphase systems of particles
(solids or liquids) dispersed in air or in other gases.
EFFECTS OF AIR POLLUTION
a) EFFECTS ON HUMANS
Our respiratory system has a number of mechanisms that help in protecting us from air pollution. Our
respiratory system has a number of mechanisms that help in protecting us from air pollution. The hair in
our nose filters out large particles. The sticky mucus in the lining of the upper respiratory tract captures
smaller particles and dissolves some gaseous pollutants. When the upper respiratory system is irritated by
pollutants sneezing and coughing expel contaminated air and mucus. Prolonged smoking or exposure to
air pollutants can overload or breakdown these natural defenses causing or contributing to diseases such
as lung cancer, asthma, chronic bronchitis and emphysema. Elderly people, infants, pregnant women and
people with heart disease, asthma or other respiratory diseases are especially vulnerable to air pollution.
Cigarette smoking is responsible for the greatest exposure to carbon monoxide. Exposure to air
containing even 0.001 percent of carbon monoxide for several hours can cause collapse, coma and even
death. As carbon monoxide remains attached to hemoglobin in blood for a long time, it accumulates and
reduces the oxygen carrying capacity of blood. This impairs perception and thinking, slows reflexes and
causes headaches, drowsiness, dizziness and nausea. Carbon monoxide in heavy traffic causes headaches,
drowsiness and blurred vision.
Sulphur dioxide irritates respiratory tissues. Chronic exposure causes a condition similar to bronchitis. It
also reacts with water, oxygen and other material in the air to form sulphur-containing acids. The acids
can become attached to particles which when inhaled are very corrosive to the lung.
Nitrogen oxides especially NO2 can irritate the lungs, aggravate asthma or chronic bronchitis and also
increase susceptibility to respiratory infections such as influenza or common colds.
Suspended particles aggravate bronchitis and asthma. Exposure to these particles over a long period of
time damages lung tissue and contributes to the development of chronic respiratory disease and cancer.
Many volatile organic compounds such as (benzene and formaldehyde) and toxic particulates (such as
lead, cadmium) can cause mutations, reproductive problems or cancer. Inhaling ozone, a component of
photochemical smog causes coughing, chest pain, breathlessness and irritation of the eye, nose and the
throat.
b) EFFECTS ON PLANTS
When some gaseous pollutants enter leaf pores they damage the leaves of crop plants. Chronic exposure
of the leaves to air pollutants can break down the waxy coating that helps prevent excessive water loss
and leads to damage from diseases, pests, drought and frost. Such exposure interferes with photosynthesis
and plant growth, reduces nutrient uptake and causes leaves to turn yellow, brown or drop off altogether.
At a higher concentration of sulphur dioxide majority of the flower buds become stiff and hard. They
eventually fall from the plants, as they are unable to flower.
Prolonged exposure to high levels of several air pollutants from smelters, coal burning power plants and
industrial units as well as from cars and trucks can damage trees and other plants.
c) EFFECTS ON MATERIALS
Every year air pollutants cause damage worth billions of rupees. Air pollutants break down exterior paint
on cars and houses. All around the world air pollutants have discoloured irreplaceable monuments,
historic buildings, marble statues, etc.
12
Downloaded From www.ktunotes.in
d) EFFECTS ON STRATOSPHERE
The upper stratosphere consists of considerable amounts of ozone, which works as an effective screen for
ultraviolet light. This region called the ozone layer extends up to 60 km above the surface of the earth.
Though the ozone is present up to 60 km its greatest density remains in the region between 20km to 25
km. The ozone layer does not consist of solely ozone but a mixture of other common atmospheric gases.
In the densest ozone layer there will be only one ozone molecule in 100,000 gas molecules. Therefore
even small changes in the ozone concentration can produce dramatic effects on life on earth.
Ozone is a form of oxygen with three atoms instead of two. It is produced naturally from the photo
dissociation of oxygen gas molecules in the atmosphere. The ozone thus formed is constantly broken
down by naturally occurring processes that maintain its balance in the ozone layer. In the absence of
pollutants the creation and breakdown of ozone are purely governed by natural forces, but the presence of
certain pollutants can accelerate the breakdown of ozone. Though it was known earlier that ozone shows
fluctuations in its concentrations which may be accompanied sometimes with a little ozone depletion, it
was only in 1985 that the large scale destruction of the ozone also called the Ozone Hole came into
limelight when some British researchers published measurements about the ozone layer.
Soon after these findings a greater impetus was given to research on the ozone layer, which convincingly
established that CFC’s were leading to its depletion. These CFCs (chloro-fluorocarbons) are extremely
stable, non-flammable, non-toxic and harmless to handle. This makes them ideal for many industrial
applications like aerosols, air conditioners, refrigerators and fire extinguishers. Many cans, which give
out foams and sprays, use CFCs. (eg: perfumes, room fresheners, etc.) CFCs are also used in making
foams for mattresses and cushions, disposable Styrofoam cups, glasses, packaging material for insulation,
cold storage etc. However their stability also gives them a long life span in the atmosphere.
Halons are similar in structure to the CFCs but contain bromine atoms instead of chlorine. They are more
dangerous to the ozone layer than CFCs. Halons are used as fire extinguishing agents as they do not pose
harm to people and equipment exposed to them during firefighting.
The CFCs and the halons migrate into the upper atmosphere after they are released. As they are heavier
than air they have to be carried by air currents up to just above the lower atmosphere and then they
slowly diffuse into the upper atmosphere. This is a slow process and can take as long as five to fifteen
years. In the stratosphere unfiltered UV-radiation severs the chemical bonds releasing chlorine from the
rest of the CFC. This attacks the ozone molecule resulting in its splitting into an oxygen molecule and an
oxygen atom.
Despite the fact that CFCs are evenly distributed over the globe, the ozone depletion is especially
pronounced over the South Pole due to the extreme weather conditions in the Antarctic atmosphere. The
presence of the ice crystals makes the Cl-O bonding easier. The ozone layer over countries like Australia,
New Zealand, South Africa and parts of South America is also depleted.
India has signed the Montreal Protocol in 1992, which aims to control the production and consumption of
Ozone Depleting Substances.
Effects of Ozone Depletion
Changes in the ozone layer have serious implications for mankind.
i) Effects on human health: Sunburn, cataract, aging of the skin and skin cancer are caused by increased
ultra-violet radiation. It weakens the immune system by suppressing the resistance of the whole body to
certain infections like measles, chicken pox and other viral diseases that elicit rash and parasitic diseases
such as malaria introduced through the skin.
ii) Food production: Ultra violet radiation affects the ability of plants to capture light energy during the
process of photosynthesis. This reduces the nutrient content and the growth of plants. This is seen
especially in legumes and cabbage.
Plant and animal planktons are damaged by ultra-violet radiation. In zooplanktons (microscopic animals)
the breeding period is shortened by changes in radiation. As planktons form the basis of the marine food
chain a change in their number and species composition influences fish and shell fish production.
13
Downloaded From www.ktunotes.in
iii) Effect on materials: Increased UV radiation damages paints and fabrics, causing them to fade faster.
iv) Effect on climate: Atmospheric changes induced by pollution contribute to global warming, a
phenomenon which is caused due to the increase in concentration of certain gases like carbon dioxide,
nitrogen oxides, methane and CFCs. Observations of the earth have shown beyond doubt that
atmospheric constituents such as water vapour, carbon dioxide, methane, nitrogen oxides and
ChloroFluoro Carbons trap heat in the form of infra-red radiation near the earth’s surface. This is known
as the ‘Greenhouse Effect’. The phenomenon is similar to what happens in a greenhouse. The glass in a
greenhouse allows solar radiation to enter which is absorbed by the objects inside. These objects radiate
heat in the form of terrestrial radiation, which does not pass out through the glass. The heat is therefore
trapped in the greenhouse increasing the temperature inside and ensuring the luxuriant growth of plants.
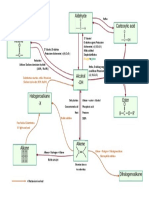
Fig. 1: Greenhouse Effect
There could be several adverse effects of global warming.
• With a warmer earth the polar ice caps will melt causing a rise in ocean levels and flooding of coastal
areas.
• In countries like Bangladesh or the Maldives this would be catastrophic. If the sea level rises by 3m,
Maldives will disappear completely beneath the waves.
• The rise in temperature will bring about a fall in agricultural produce.
• Changes in the distribution of solar energy can bring about changes in habitats. A previously productive
agricultural area will suffer severe droughts while rains will fall in locations that were once deserts. This
could bring about changes in the species of natural plants, agricultural crops, insects, livestock and micro-
organisms.
• In the polar regions temperature rises caused by global warming would have disastrous effects. Vast
quantities of methane are trapped beneath the frozen soil of Alaska. When the permafrost melts the
methane that will be released can accelerate the process of global warming.
14
Downloaded From www.ktunotes.in
Pollutants Sources Effects Prevention and Permissible Levels
controil NAAQS standards
in India
Ozone (O3) Gas formed It Causes irritation Reduce motor Permissible
indirectly, by the in respiratory vehicle concentration of
process of tract, It can trigger Volatile organic ozone on hourly
photochemical air a variety of health compounds weighted average
pollution. Formed problems (VOCs) and value of 180
when Volatile including chest nitrogen oxide µg/m3 for
organic pain, coughing, emissions through residential areas.
compounds throat irritation, emissions
(VOCs) and and congestion, It standards,
nitrogen oxides can worsen reformulated
react in the bronchitis, fuels,
presence of emphysema, and inspections
sunlight. VOC asthma, It can programs and
sources include reduce lung reduced vehicle
any source that function and use.
burns fuels, (e.g., inflame the linings Limit VOC
gasoline, natural of the lungs, emissions from
gas, wood, oil) Repeated commercial
solvents, exposure may operations and
petroleum permanently scar consumer
processing and lung tissue. products. Limit
storage and VOC and
pesticides. NOx emissions
from industrial
sources such as
power plants and
refineries.
Conserve energy.
Respirable Usually emitted Increased Control Dust Permissible
Particulate Matter directly as a result Respiratory Sourses, Industrial
(PM10) of mechanical Disease, Lung particulate
processes that Damage, Cancer, emissions, wood
crush or grind Premature Death, burning stoves and
larger particles, or nonfatal heart fire places
by resuspension of attacks, irregular
dusts in the heartbeat,
atmosphere. Road aggravated
Dust, Windblown asthma, decreased
Dust (Agriculture) lung function, and
and Construction increased
(Fireplaces), also respiratory
15
Downloaded From www.ktunotes.in
formed from other symptoms, such
pollutants (acid as irritation of
rain, NOx, the airways,
SOx, organics). coughing or
Incomplete difficulty
combustion of any breathing
fuel. Reduced
Visibility,
Surface Soiling
16
Downloaded From www.ktunotes.in
You might also like
- Bachelor of Engineering - Semester I (Civil and Mechanical Branch) Academic Year-2017-18Document39 pagesBachelor of Engineering - Semester I (Civil and Mechanical Branch) Academic Year-2017-18VijendraNo ratings yet
- Define Environment and Factor of Environmental PollutionDocument6 pagesDefine Environment and Factor of Environmental PollutionAdan Hooda100% (1)
- As Chemistry Organic MindmapDocument1 pageAs Chemistry Organic MindmapDương Thị Ngọc HiềnNo ratings yet
- Chemical Formula List For Class 10Document12 pagesChemical Formula List For Class 10The Coding World100% (3)
- Science Question BankDocument291 pagesScience Question BankA. PradeepaNo ratings yet
- Chemical Calculations: Mass of Cucl .2H O Molar Mass of Cucl .2H O 3.42 64 + (2 ! 35.5) + (2 ! 18)Document5 pagesChemical Calculations: Mass of Cucl .2H O Molar Mass of Cucl .2H O 3.42 64 + (2 ! 35.5) + (2 ! 18)khalil rehmanNo ratings yet
- Stepan Vehicle Care BrochureDocument5 pagesStepan Vehicle Care BrochureRafael Santo SilvaNo ratings yet
- Types of PollutionDocument11 pagesTypes of PollutionAnonymous yZuCUO75% (24)
- The Air Environment: Engr. Allyzza Nichole Velasco, Cie, Aae Industrial Engineering Department, UBDocument51 pagesThe Air Environment: Engr. Allyzza Nichole Velasco, Cie, Aae Industrial Engineering Department, UBUlasir CometaNo ratings yet
- EDAPLAN ® 490 - MunzingDocument4 pagesEDAPLAN ® 490 - MunzingLong An Đỗ0% (1)
- Types of PollutionDocument25 pagesTypes of Pollutionlilyxoxo1812No ratings yet
- Shoaib Final PDF of Air PollutionDocument11 pagesShoaib Final PDF of Air PollutionWaseem AkramNo ratings yet
- Environmental Chemistry CHM 115Document14 pagesEnvironmental Chemistry CHM 115Venom BlackNo ratings yet
- UNIT5 Environmental PollutionDocument24 pagesUNIT5 Environmental Pollutionhimanshi harsanaNo ratings yet
- Unit 5 EVSDocument47 pagesUnit 5 EVSyash02005No ratings yet
- EnglishDocument80 pagesEnglishShivam singhNo ratings yet
- InPc Unit 1 NotesDocument10 pagesInPc Unit 1 NotesA SrikrishnanNo ratings yet
- Pollutions 15042023Document39 pagesPollutions 15042023Prashant ChaudhryNo ratings yet
- Environmental Pollution and Responsible FactorsDocument7 pagesEnvironmental Pollution and Responsible FactorsShyam Sundar100% (1)
- Lec#01 Environment Pollution ControlDocument28 pagesLec#01 Environment Pollution ControlYasir ShaikhNo ratings yet
- Introduction To Industrial PollutionDocument8 pagesIntroduction To Industrial PollutionMohit D MoreNo ratings yet
- Environmental Pollution & Sold Waste Management: UNIT-3Document26 pagesEnvironmental Pollution & Sold Waste Management: UNIT-3sarfarzNo ratings yet
- EnvironmentallawDocument65 pagesEnvironmentallawrahul singhNo ratings yet
- PollutionDocument9 pagesPollutionTechnical GodNo ratings yet
- Lectur 5Document52 pagesLectur 5fidamuqadas123No ratings yet
- Water, Air, and Soil Environment PollutionDocument3 pagesWater, Air, and Soil Environment PollutionJohn Marvin AbelardeNo ratings yet
- Environmental PollutionDocument12 pagesEnvironmental Pollutionyochankulung321No ratings yet
- Evs Assignment1Document23 pagesEvs Assignment1Harsh AgarwalNo ratings yet
- Environemntal PollutionDocument89 pagesEnvironemntal PollutionPrincess Jean L. GalabinNo ratings yet
- Lecture Note CH 1Document80 pagesLecture Note CH 1kidus sileshNo ratings yet
- Moiz Naveed CE 590950: Environment Pollution (3676)Document21 pagesMoiz Naveed CE 590950: Environment Pollution (3676)Moix KhanNo ratings yet
- Basics of Environmental LawDocument9 pagesBasics of Environmental Lawxakij19914No ratings yet
- Environmental PollutionDocument19 pagesEnvironmental PollutionKolkuluri BharghavNo ratings yet
- Air Pollution Is The Introduction ofDocument4 pagesAir Pollution Is The Introduction ofGuru MurthyNo ratings yet
- Environmental PollutionsDocument6 pagesEnvironmental PollutionsSATUR LABRADURNo ratings yet
- Impact of Industrialization & Modernization On EnvironmentDocument98 pagesImpact of Industrialization & Modernization On EnvironmentYogesh ShindeNo ratings yet
- Environmental PollutionDocument19 pagesEnvironmental Pollutionchinedumpeters31No ratings yet
- Environmental PollutionDocument42 pagesEnvironmental PollutionGood DoogNo ratings yet
- Air Pollution:-: Serious Environmental ProblemDocument11 pagesAir Pollution:-: Serious Environmental ProblemAbhipsa BisiNo ratings yet
- PollutionDocument2 pagesPollutionhardikpawarNo ratings yet
- It Is Made Up of 78% Nitrogen, 21% Oxygen and 1% Other Substances (Including Water Vapor)Document11 pagesIt Is Made Up of 78% Nitrogen, 21% Oxygen and 1% Other Substances (Including Water Vapor)DroseilvanNo ratings yet
- محاضرات تلوث بيئيDocument63 pagesمحاضرات تلوث بيئيمصطفى العباديNo ratings yet
- Environmental Pollution: Primary and Secondary Air PollutantsDocument10 pagesEnvironmental Pollution: Primary and Secondary Air PollutantsWinsomeboi HoodlegendNo ratings yet
- Environmental Pollution - 231026 - 092559Document37 pagesEnvironmental Pollution - 231026 - 092559iam prajapatiNo ratings yet
- PollutionDocument10 pagesPollutionlohith.ganeshkumarNo ratings yet
- Pollutants: Chapter No 3 Chemistry of PollutantsDocument13 pagesPollutants: Chapter No 3 Chemistry of PollutantsM. Salih hayat KhanNo ratings yet
- Q4 Long Test Reviewer EnviDocument5 pagesQ4 Long Test Reviewer EnviAlma TatelNo ratings yet
- Unit-4: Environmental PollutionDocument29 pagesUnit-4: Environmental PollutionNeetika ChawlaNo ratings yet
- Unit 5: Environmental PollutionDocument45 pagesUnit 5: Environmental PollutionNicholas WhiteNo ratings yet
- PollutionDocument10 pagesPollutionNooril MoujudhuNo ratings yet
- Environmental Management Shivjeet Mam-1Document5 pagesEnvironmental Management Shivjeet Mam-1Arshdeep SinghNo ratings yet
- UNIT 2 Sustainable EngineeringDocument16 pagesUNIT 2 Sustainable EngineeringSrikanth VNo ratings yet
- Pollution and Its TypesDocument46 pagesPollution and Its Typesmuhtamimkhan276No ratings yet
- Sorces of Air Pollution Air PollutionDocument8 pagesSorces of Air Pollution Air PollutionRohan ChauguleNo ratings yet
- Air Pollution: Author A.G.Naveen Kumar Co-Author A.Aswin KumarDocument12 pagesAir Pollution: Author A.G.Naveen Kumar Co-Author A.Aswin KumarRaghu NandhanNo ratings yet
- Environmental Pollution NotesDocument17 pagesEnvironmental Pollution NotesHimanish KoyalkarNo ratings yet
- PollutionDocument6 pagesPollutionAkanksha PashineyNo ratings yet
- FinalsACT1 SentinDocument8 pagesFinalsACT1 SentinRonielyn SentinNo ratings yet
- Air Pollution v2.0Document32 pagesAir Pollution v2.0JYCR LCSMNo ratings yet
- Air Toxics and Acid DepositionDocument17 pagesAir Toxics and Acid DepositionNehala RaufNo ratings yet
- 12 K Pandya DR JigneshDocument4 pages12 K Pandya DR Jigneshkemalusman383No ratings yet
- Air, Noise and Soil PollutionDocument7 pagesAir, Noise and Soil PollutionjunaidNo ratings yet
- Air Pollution Causes: Anthropogenic Sources (Human Activity) Mostly Related To Burning Different Kinds ofDocument6 pagesAir Pollution Causes: Anthropogenic Sources (Human Activity) Mostly Related To Burning Different Kinds ofSarvesh JaiswalNo ratings yet
- Environment & EcologyDocument10 pagesEnvironment & EcologySRIKANT KUMAR NAYAKNo ratings yet
- Say No To PollutionDocument12 pagesSay No To PollutionAbeera AdanNo ratings yet
- TRack MaintaneneceDocument92 pagesTRack MaintaneneceVishnu WdrNo ratings yet
- Reg No.: - Name: - : MarksDocument1 pageReg No.: - Name: - : MarksVishnu WdrNo ratings yet
- Geosynthetic Engineering - Basic ConceptsDocument9 pagesGeosynthetic Engineering - Basic ConceptsVishnu WdrNo ratings yet
- Septic Tank EstimationDocument9 pagesSeptic Tank EstimationVishnu WdrNo ratings yet
- Chlorides ManualDocument3 pagesChlorides ManualVishnu WdrNo ratings yet
- Department of Civil Engineering College of Engineering TrivandrumDocument2 pagesDepartment of Civil Engineering College of Engineering TrivandrumVishnu WdrNo ratings yet
- Wle Se Im: Teu) Ex Be A and Olhre Bransfsrma BooDocument8 pagesWle Se Im: Teu) Ex Be A and Olhre Bransfsrma BooVishnu WdrNo ratings yet
- QSV Module 2Document13 pagesQSV Module 2Vishnu WdrNo ratings yet
- EIA MODULE 3-Ktunotes - inDocument30 pagesEIA MODULE 3-Ktunotes - inVishnu WdrNo ratings yet
- Cu (II) ACUTE TOXICITY TO THE ROTIFERDocument5 pagesCu (II) ACUTE TOXICITY TO THE ROTIFERAndresPortaNo ratings yet
- 1.14.5 Gas Chromatography: The International Pharmacopoeia - Ninth Edition, 2019Document4 pages1.14.5 Gas Chromatography: The International Pharmacopoeia - Ninth Edition, 2019Dinesh PatoleNo ratings yet
- TDS Vinkocide Eco enDocument3 pagesTDS Vinkocide Eco enYessikaNo ratings yet
- Influence of Reaction Operation Conditions On The Final Properties of High Impact Polystyrene (HIPS) PDFDocument13 pagesInfluence of Reaction Operation Conditions On The Final Properties of High Impact Polystyrene (HIPS) PDFGhina Fatikah SalimNo ratings yet
- Atp Adp CycleDocument34 pagesAtp Adp CycleJepoy dizon Ng Tondo Revengerz gangNo ratings yet
- Chemical and Food Engineering Department Introduction To EngineeringDocument16 pagesChemical and Food Engineering Department Introduction To EngineeringJoshua Miguel BunoNo ratings yet
- CB307 IntroductionDocument52 pagesCB307 IntroductionSiddhant SoymonNo ratings yet
- Unit IV PPT - Chemical BondingDocument68 pagesUnit IV PPT - Chemical BondingYash TajaneNo ratings yet
- Biosensing Based On Pencil Graphite ElectrodesDocument40 pagesBiosensing Based On Pencil Graphite ElectrodesaczirokNo ratings yet
- Gpat 2018Document24 pagesGpat 2018madanjitenderNo ratings yet
- Matching: Match Each Item With The Correct Statement BelowDocument16 pagesMatching: Match Each Item With The Correct Statement BelowwallaNo ratings yet
- A Review On Wet Granulation TechnologyDocument20 pagesA Review On Wet Granulation TechnologyAdi permadiNo ratings yet
- Safety Data Sheet Rust Remover: 1 Product and Company IdentificationDocument5 pagesSafety Data Sheet Rust Remover: 1 Product and Company Identificationafryan azhar kurniawanNo ratings yet
- XRD Analysis of Clay Minerals VOL 2 Practical Identification TheoryDocument45 pagesXRD Analysis of Clay Minerals VOL 2 Practical Identification Theoryjuan diego gomez quinteroNo ratings yet
- Quality Control Interview Questions For PharmaDocument14 pagesQuality Control Interview Questions For PharmaAdamNo ratings yet
- Lipids: Biochemistry LaboratoryDocument46 pagesLipids: Biochemistry LaboratoryZiaNo ratings yet
- Lab Report Environmental Engineering 2 (CEL304)Document40 pagesLab Report Environmental Engineering 2 (CEL304)Shivang KumarNo ratings yet
- Metabolic Engineering of Escherichia Coli For The Biosynthesis of Alpha PineneDocument10 pagesMetabolic Engineering of Escherichia Coli For The Biosynthesis of Alpha PineneMaruf MuhammadNo ratings yet
- Manufacturing of Glass 1Document21 pagesManufacturing of Glass 1MonishNo ratings yet
- ISC Chemistry 23-24Document10 pagesISC Chemistry 23-24jeeileena02No ratings yet
- Immobilization of EnzymesDocument2 pagesImmobilization of EnzymesDevlina SenguptaNo ratings yet
- Spectro RamanDocument6 pagesSpectro RamanSampada, Astrologer and Vastu Spl. SSBNo ratings yet
- Solid Dosage Forms - CapsulesDocument14 pagesSolid Dosage Forms - CapsulesHellcroZNo ratings yet
- 1 - Stoichiometry-01 - TheoryDocument42 pages1 - Stoichiometry-01 - TheoryRaju SinghNo ratings yet