Professional Documents
Culture Documents
Sit (En571) HW3
Sit (En571) HW3
Uploaded by
kelsi chanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sit (En571) HW3
Sit (En571) HW3
Uploaded by
kelsi chanCopyright:
Available Formats
EN 571 Homework 3
Solve the problems in MATLAB Grader (https://grader.mathworks.com/)
March 7, 2021
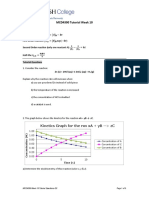
1. Problem
A general species A at the initial concentration (ca,0 ) of 1 mol/L is introduced into a reactor at 40 ◦ C, where
it reacts to the product R according to the irreversible single-step elementary reaction:
A −→ B.
The concentration of A (ca ) in the reactor is monitored at various times and the results are:
Table 1: Concentration of A over time.
time, min 0 100 200 300 400
ca , mol/m3 1000 500 333 250 200
1) Write the rate law of the reaction considering a rate constant equal to 0.0052 1/min; 2) half-life time
(t = t 21 ) of the reaction; 2) the conversation of A after 6 hours from the beginning of the reaction; 3)
considering an activation energy (Ea ) of 4,500 J/mol, which is the rate constant at 80 ◦ C?
2. Problem
In the water softening reactor, the following precipitation reaction occurs:
A + B ←→ C.
The forward rate constant (k1 ) is equal to 0.01 mol−1 min−1 and the solubility product is equal to 102 . The
initial concentrations for A, B, and C are, respectively: 1, 2, and 0 mol/L. Determine: 1) the order of the
overall reaction; 2) the rate constant of the dissolution reaction (i.e., backward reaction); 3) write the rate
law for the production of C considering limiting concentration of A and B, solve it numerically; and 4) the
amount of precipitate after 2 hours of reaction assuming 1 L reactor.
You might also like
- Chemical Kinetics-I: Part - I: Subjective QuestionsDocument34 pagesChemical Kinetics-I: Part - I: Subjective Questionshorn blowNo ratings yet
- CHNG 3004 - 2019-2020 AssignmentsDocument26 pagesCHNG 3004 - 2019-2020 AssignmentsXheikhKaleem100% (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Problem Set ODocument19 pagesProblem Set OnimboNo ratings yet
- Tutorial 2 StudentDocument6 pagesTutorial 2 StudentIrsyad KamilNo ratings yet
- Rr320802chemicalreactionengineeringiDocument8 pagesRr320802chemicalreactionengineeringiSanthosh KumarNo ratings yet
- Kinetics Ans Key Master FileDocument10 pagesKinetics Ans Key Master FileJOANA RHEA SAGPAEYNo ratings yet
- t11 Reaction Kinetics 19-26Document7 pagest11 Reaction Kinetics 19-26lorraine_cuaNo ratings yet
- Tutorial For Chapter 1Document3 pagesTutorial For Chapter 1Thurgah VshinyNo ratings yet
- Che 125: Chemical Reaction Engineering IDocument2 pagesChe 125: Chemical Reaction Engineering IJelor GallegoNo ratings yet
- Tutorial 3 QuestionDocument3 pagesTutorial 3 Questionnur hidayatiNo ratings yet
- Chemical KineticsDocument3 pagesChemical KineticsakritiNo ratings yet
- R (DC /DT) 0.2 Mol L C: ASSIGNMENT #2 - Reaction Kinetics 2Document2 pagesR (DC /DT) 0.2 Mol L C: ASSIGNMENT #2 - Reaction Kinetics 2AndreNo ratings yet
- GZB - Xii - WS-8 - Chemistry - Chemical Kinetics - OctoberDocument3 pagesGZB - Xii - WS-8 - Chemistry - Chemical Kinetics - OctoberSaman PanwarNo ratings yet
- 1.chemical KineticsDocument24 pages1.chemical KineticsVinod AgrawalNo ratings yet
- r05310805 Chemical Reaction Engineering IDocument8 pagesr05310805 Chemical Reaction Engineering ISrinivasa Rao GNo ratings yet
- Exercise Chapter 5Document8 pagesExercise Chapter 5Arics ChiengNo ratings yet
- Chemical KineticsDocument3 pagesChemical Kineticsnimitsigotiya2No ratings yet
- Mid Term General Chem II Fall 2001Document6 pagesMid Term General Chem II Fall 2001dr.ibrahimsalemvpNo ratings yet
- Assignment 2Document4 pagesAssignment 2Yi Hong LowNo ratings yet
- Assignment #4 Chemical Reaction Engineering: WarningDocument2 pagesAssignment #4 Chemical Reaction Engineering: Warningvrutu tapirNo ratings yet
- Ki KBR H C Ki BR H C: Oducts B ADocument2 pagesKi KBR H C Ki BR H C: Oducts B AnaverfallNo ratings yet
- Homework 2 QuestionsDocument1 pageHomework 2 QuestionsTouqeer iqbalNo ratings yet
- Chee4367 HW051231Document2 pagesChee4367 HW051231kimhoang_16927574No ratings yet
- 12th Grade Chemical Kinetics WorhshhetDocument1 page12th Grade Chemical Kinetics WorhshhetAmen RaipurNo ratings yet
- Kinetics & Photochemistry Tutorial ProblemsDocument4 pagesKinetics & Photochemistry Tutorial ProblemsAmbuj Yadav 4-Year B.Tech. Chemical EngineeringNo ratings yet
- Chemical Kinetics AssignmentDocument10 pagesChemical Kinetics Assignmentmanglaaayush21No ratings yet
- R09 Set No. 2Document8 pagesR09 Set No. 2Shakoor MalikNo ratings yet
- Chemical KineticsDocument4 pagesChemical KineticsShubhankar SinhaNo ratings yet
- JAB-TALLER 3er PARCIAL IRQ-2020-1Document5 pagesJAB-TALLER 3er PARCIAL IRQ-2020-1JESSICA PAOLA TORO VASCONo ratings yet
- nr320802 Chemical Reaction Engineering IDocument2 pagesnr320802 Chemical Reaction Engineering ISRINIVASA RAO GANTANo ratings yet
- 3 QP Chemical KineticsDocument4 pages3 QP Chemical KineticsSnehit RajNo ratings yet
- CRE - Diagnostic Exam (USA)Document2 pagesCRE - Diagnostic Exam (USA)Kuo SarongNo ratings yet
- Big Idea 4 AnswersDocument4 pagesBig Idea 4 AnswersSreeyaNo ratings yet
- XII - Revision Sheet - 2 - ChemistryDocument3 pagesXII - Revision Sheet - 2 - ChemistryVipin VNo ratings yet
- CRE Assignment - 1 (Kinetics - Rate Constant and Mole Balance Over Chemical Reactions With Answer Keys)Document6 pagesCRE Assignment - 1 (Kinetics - Rate Constant and Mole Balance Over Chemical Reactions With Answer Keys)Ankush GuptaNo ratings yet
- Revision QuestionDocument2 pagesRevision QuestionBilal AhmadNo ratings yet
- 1 - Prob Kinet 11-12 1-13 EnglishDocument4 pages1 - Prob Kinet 11-12 1-13 EnglishYenNo ratings yet
- Exam I Sem I 2011 12 Cheng 323Document7 pagesExam I Sem I 2011 12 Cheng 323Faisal MumtazNo ratings yet
- Chemistry (Maninagar-Target) Section-I (Only One Option Correct)Document4 pagesChemistry (Maninagar-Target) Section-I (Only One Option Correct)Rajeev GangwarNo ratings yet
- CLIP - Chemical Kinetics PDFDocument4 pagesCLIP - Chemical Kinetics PDFAman JaiswalNo ratings yet
- CHEN30001 Tutorial 1Document2 pagesCHEN30001 Tutorial 1Ricky Pompii ShoeNo ratings yet
- Deodhar PhysicsDocument4 pagesDeodhar PhysicsAditya MoreNo ratings yet
- Chapter 14 HOME WITH ANSDocument7 pagesChapter 14 HOME WITH ANSSerpicoNo ratings yet
- ChEMICAL KINETICS - QUESTIONSDocument3 pagesChEMICAL KINETICS - QUESTIONSChhabi YadavNo ratings yet
- 2018l19 Final Exam With SolutionDocument13 pages2018l19 Final Exam With SolutionGAMERS OF KUWAITNo ratings yet
- Cinetica WagnerDocument11 pagesCinetica WagnerJesus Manuel Yallerco VenegasNo ratings yet
- MCD4390 Week 10 Tutorial QuestionsDocument5 pagesMCD4390 Week 10 Tutorial QuestionsGabbar100% (1)
- Delhi Public School: Nacharam/ Mahendra Hills/ NadergulDocument3 pagesDelhi Public School: Nacharam/ Mahendra Hills/ Naderguleeshwar saagarNo ratings yet
- CRE I Assignment - 250919Document11 pagesCRE I Assignment - 250919UpanyaaNo ratings yet
- Worksheet On CH TWODocument3 pagesWorksheet On CH TWOfikadubiruk87No ratings yet
- Chemical Reaction Engineering (CHE-306) RCS (Makeup)Document2 pagesChemical Reaction Engineering (CHE-306) RCS (Makeup)Ishan RatnakarNo ratings yet
- Tutorial 5drtuhDocument2 pagesTutorial 5drtuhFikrie MuhdNo ratings yet
- Chosen Problems of Chapter 3-QuestionsDocument6 pagesChosen Problems of Chapter 3-QuestionsBilal AhmedNo ratings yet
- 5bfd1a25-a358-45a3-b994-02540a001a19Document2 pages5bfd1a25-a358-45a3-b994-02540a001a19Student KeekNo ratings yet
- CRE Assignment - 1Document3 pagesCRE Assignment - 1Rishikesh100% (1)
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)