Professional Documents
Culture Documents
Condiciones de Analisis de Gluten Por HPLC
Uploaded by
xacvierCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Condiciones de Analisis de Gluten Por HPLC
Uploaded by
xacvierCopyright:
Available Formats
Chemicals and reagents.
Solvents and reagents used for the present studies were analytical quality or better, unless described
differently. For the preparation of HPLC solvents, trifluoroacetic acid (TFA) of sequencing grade (Fluka 91699) and acetonitrile
(ACN) of gradient grade (Merck 100030) or of preparative grade (Merck 113358) were used. Bovine serum albumin (BSA
standard) was purchased from Serva (11920), and propyl-4-hydroxybenzoate from Fluka (54790).
Solvents. A1: 0.4 mol/L of NaCl + 0.067 mol/L of HKNaPO 4 (pH 7.6); A2: 0.4 mol/L of NaCl. B1: 60% (v/v) ethanol (EtOH); B2:
50% (v/v) 1-propanol (1-PrOH); B3: 50% (v/v) 2-propanol (2-PrOH). C1: 50% (v/v) 1-PrOH + 2 mol/L of urea + 0.05 mol/L of
Tris-HCl (pH 7.5) + 1% (w/v) dithioerythritol (DTE) under nitrogen; C2: 50% (v/v) 2-PrOH + 0.08 mol/L of Tris-HCl (pH 8.0) +
1% (w/v) DTE under nitrogen.
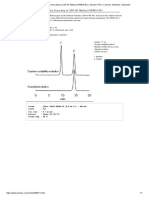
RP-HPLC. Routine RP-HPLC of the filtered protein fractions extracted from flour and the different protein standards was
performed using a Beckman instrument (solvent module 126, System Gold software); Nucleosil 300-5 C8 column material
(Machery-Nagel 71265) packed into a 4.6- × 240-mm column by an HPLC packing instrument (Shandon) under a pressure of 450
bar (which was much cheaper than a commercially available column); column temperature was 50°C; sample loop was 2 mL;
injection was 200 mL of albumins and globulins, 50 or 80 mL of gliadins, and 100 or 150 mL of glutenin subunits; 0.1% (v/v) TFA
(500 mL) was injected before and after sample injection. Elution system I: A) TFA (0.1%, v/v); B) ACN (preparative grade) + TFA
(99.9/0.1%, v/v). Linear gradients: 0 min 20% B, 20 min 60% B (albumins-globulins); 0 min 28% B, 30 min 56% B (gliadins,
glutenin subunits, gliadin standard, LMW standard, HMW standard); 0 min 39.5% B, 20 min 41.0% B (BSA standard). Flow rate
was 1.0 mL/min; detection was by UV absorbance at 210 nm. After each run, the column was cleaned with 90% B (5 min) and
equilibrated with the starting B concentration (10 min).
For the analysis of HMW subunits, changes included column temperature of 53°C and injection of 250 mL of the glutenin extract.
Elution system II: A) ACN (15%, v/v) + 2-PrOH (10%, v/v) + TFA (0.1%, v/v); B) ACN (30%, v/v) + 2-PrOH (8%, v/v) + TFA
(0.1%, v/v). Linear gradients: 0 min 46% B, 60 min 71% B, 61–65 min 100% B. Flow rate was 0.8 mL. Other conditions tested
included: Nucleosil 300-5 C 18 column material (Machery-Nagel 71252); gradient grade ACN; column temperature 70°C.
Gradients: 0 min 24% B, 50 min 56% B, or 0 min 27% B, 20 min 55% B (gliadins, glutenin subunits). UV absorbance was at 220
nm. The integration procedure was automatically handled by the software. The baseline was adjusted to zero when injector was
moved to inject position. Integration was suppressed for the first part of the chromatograms (negative and positive solvent peaks).
The elution borders of the protein groups were set to absorbance minima between specific peaks. T he absorbance spectra of eluted
proteins were determined by a diode-array detector (Beckman module 168). Column quality was tested with propyl-4-
hydroxybenzoate (1.5 mg dissolved in 30 mL of methanol) eluted with 50% B of elution system I or II; peak width at half peak
height was taken as quality criterion.
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (589)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5806)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- A751Document6 pagesA751SUBHOMOY100% (1)
- Quantitative Determination of Total Hardness in Drinking Water by Complexometric Edta TitrationDocument5 pagesQuantitative Determination of Total Hardness in Drinking Water by Complexometric Edta TitrationJoNo ratings yet
- AqcGuidance SANTE 2019 12682Document52 pagesAqcGuidance SANTE 2019 12682xacvierNo ratings yet
- Vitamin A and E in Infant Formula and Adult - Pediatric Nutritional Formula - 2012.09Document6 pagesVitamin A and E in Infant Formula and Adult - Pediatric Nutritional Formula - 2012.09Yamasi MakassarNo ratings yet
- Environmental Protection Agency Pt. 136, App. B: T 6-A E C C / 'Document4 pagesEnvironmental Protection Agency Pt. 136, App. B: T 6-A E C C / 'xacvierNo ratings yet
- CXS 234e PDFDocument80 pagesCXS 234e PDFnhonducNo ratings yet
- Analysis of Sorbitol According To USP-NF Method (SP0810 8C) - Shodex - HPLC Columns, Detectors, StandardsDocument1 pageAnalysis of Sorbitol According To USP-NF Method (SP0810 8C) - Shodex - HPLC Columns, Detectors, StandardsxacvierNo ratings yet
- Lista AOACDocument2 pagesLista AOACxacvierNo ratings yet
- Determination of Sugar Alcohols in Confectioneries byDocument6 pagesDetermination of Sugar Alcohols in Confectioneries byxacvierNo ratings yet
- AOAC 995.05 Vitamina DDocument3 pagesAOAC 995.05 Vitamina DxacvierNo ratings yet
- Bell Et Al 2006 Nutrient Losses and Gains EroFIR 2006 D1.5.5Document66 pagesBell Et Al 2006 Nutrient Losses and Gains EroFIR 2006 D1.5.5xacvierNo ratings yet
- Condiciones de Analisis de Gluten Por HPLCDocument1 pageCondiciones de Analisis de Gluten Por HPLCxacvierNo ratings yet
- Jurnal Analisis Stabilitas Lidokain HCLDocument11 pagesJurnal Analisis Stabilitas Lidokain HCLMusfira Dewy SuardiNo ratings yet
- 2.protein Primary StructureDocument83 pages2.protein Primary Structuremitchellpearson97No ratings yet
- TKN Determination in Water and Waste Water: Application Note No. 191/2015Document9 pagesTKN Determination in Water and Waste Water: Application Note No. 191/2015Alessandro BisbanoNo ratings yet
- 10 Science NCERT Solutions Chapter 2 Page 25Document2 pages10 Science NCERT Solutions Chapter 2 Page 25Anita GargNo ratings yet
- A Level Acid-Base Theory, Lewis Acids and Bases, Bronsted-Lowry Proton Theory GCDocument7 pagesA Level Acid-Base Theory, Lewis Acids and Bases, Bronsted-Lowry Proton Theory GCvarunvijay89No ratings yet
- UntitledDocument4 pagesUntitledhdawgNo ratings yet
- Ocratoxina en CafeDocument4 pagesOcratoxina en Cafenabucodonossor24No ratings yet
- Solutions - DPP 03 (Of Lec 04) - Lakshya JEE 2024Document2 pagesSolutions - DPP 03 (Of Lec 04) - Lakshya JEE 2024Rishi SinghalNo ratings yet
- Hydrolysis of 1,3,5-Tris (2-Hydroxyethyl) Hexahydro-S-Triazine and Its Reaction With H2S - 2001Document4 pagesHydrolysis of 1,3,5-Tris (2-Hydroxyethyl) Hexahydro-S-Triazine and Its Reaction With H2S - 2001FSBollNo ratings yet
- The Characterization of 2 - (3-Methoxyphenyl) - 2 - (Ethylamino) Cyclohexanone (Methoxetamine)Document15 pagesThe Characterization of 2 - (3-Methoxyphenyl) - 2 - (Ethylamino) Cyclohexanone (Methoxetamine)0j1u9nmkv534vw9vNo ratings yet
- CH 03Document34 pagesCH 03Waleed AbukeshekNo ratings yet
- Simultaneous Estimation of Rabeprazole and Domperidone in Dosage Forms by RP - HPLCDocument3 pagesSimultaneous Estimation of Rabeprazole and Domperidone in Dosage Forms by RP - HPLCBK RegulatoryNo ratings yet
- Simultaneous HPLC Analysis of Betamethasone and Clotrimazole in Cream Formulation PDFDocument4 pagesSimultaneous HPLC Analysis of Betamethasone and Clotrimazole in Cream Formulation PDFNájla KassabNo ratings yet
- MaduramicinaDocument18 pagesMaduramicinaJury Jasbleidy Ñungo MorenoNo ratings yet
- Experiment 1 Standardization of Acid and Base SolutionDocument6 pagesExperiment 1 Standardization of Acid and Base SolutionMarco AdenNo ratings yet
- Lab 3Document10 pagesLab 3Rahul Goel0% (1)
- CH 5 Measurement Procedures and Calculations in SpectrophotoDocument29 pagesCH 5 Measurement Procedures and Calculations in Spectrophototagele hunegnawNo ratings yet
- Practical 14 Iron Wool by Redox TitrationDocument4 pagesPractical 14 Iron Wool by Redox TitrationAngnes PoliskaNo ratings yet
- M SC Bangalore University SyllabusDocument95 pagesM SC Bangalore University Syllabusche911No ratings yet
- Knauer - Food PreservativesDocument1 pageKnauer - Food PreservativesMaryanta HadisudiroNo ratings yet
- Method Development and Validation of Paracetamol Drug by RP-HPLC 1Document7 pagesMethod Development and Validation of Paracetamol Drug by RP-HPLC 1Anonymous ncDgoMONo ratings yet
- Chapter 5 PDFDocument11 pagesChapter 5 PDFJun Elbert JaboliNo ratings yet
- Gas Absorption ExperimentDocument6 pagesGas Absorption Experimentemre06istNo ratings yet
- Record 12A: Measuring PH To Validate Your Acidification ProcessDocument2 pagesRecord 12A: Measuring PH To Validate Your Acidification ProcessJyot SoodNo ratings yet
- Purity of Salicylic Acid: Aicdx Base Titration by Automatic Potentiometric TitratorDocument4 pagesPurity of Salicylic Acid: Aicdx Base Titration by Automatic Potentiometric TitratorJinorri WilsonNo ratings yet
- Kjeldahl Lab Report Chem 331Document4 pagesKjeldahl Lab Report Chem 331Claude Bernard Jean-Guillaume40% (5)
- Calculations Involving Solutions (2) : AS Worksheet 1.2Document2 pagesCalculations Involving Solutions (2) : AS Worksheet 1.2KoleksiArkibNo ratings yet
- Quantifying Algal BiomassDocument3 pagesQuantifying Algal BiomassyuwonoyaoNo ratings yet