Professional Documents
Culture Documents
AIIMS COVID Algorithm Ver 2.0
Uploaded by
jimjose antonyOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
AIIMS COVID Algorithm Ver 2.0
Uploaded by
jimjose antonyCopyright:
Available Formats
AIIMS, New Delhi
CLINICAL GUIDANCE FOR MANAGEMENT OF COVID-19 (Version 2.0)
22nd April 2021
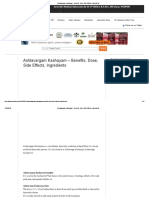
COVID-19 patient
Mild disease Moderate disease Severe disease
Upper respiratory tract symptoms Any one of: Any one of:
(&/or fever) WITHOUT shortness 1. Respiratory rate > 24 /min 1. Respiratory rate > 30 /min
of breath or hypoxia 2. SpO2 < 93% on room air 2. SpO2 < 90% on room air
Home Isolation ADMIT IN WARD ADMIT IN ICU
Oxygen Support: Respiratory support
✓ Contact & droplet precautions, strict hand ➢ Target SpO2: 92-96% (88-92% in patients with COPD) • Consider use of NIV (Helmet or face mask interface depending
hygiene
on availability)/HFNC in patients with increasing oxygen
✓ Symptomatic management (hydration, anti- ➢ Preferred devices for oxygenation: non-rebreathing face mask
pyretics, anti-tussive)
requirement, if work of breathing is LOW
✓ Stay in contact with treating physician • Intubation should be prioritized in patients with high work of
➢ Awake proning should be encouraged in all patients who are
breathing /if NIV is not tolerated ^^
requiring supplemental oxygen therapy (sequential position changes
• Seek immediate medical attention if: every 1-2 hours) • Use conventional ARDSnet protocol for ventilatory management
o Difficulty in breathing
o High grade fever/severe cough, particularly if Anti-inflammatory or immunomodulatory therapy Anti-inflammatory or immunomodulatory therapy
lasting for >5 days ➢ Inj. Methylprednisolone 0.5 to 1 mg/kg in 2 divided doses (or an • Inj Methylprednisolone 1 to 2mg/kg IV in 2 divided doses (or an
o A low threshold to be kept for those with any equivalent dose of dexamethasone) usually for a duration of 5 to 10 equivalent dose of dexamethasone) usually for a duration 5 to
of the high-risk features* days 10 days
❖ Peripheral oxygen saturation (by applying an ➢ Patients may be initiated or switched to oral route if stable and/or
SpO2 probe to fingers) should be monitored improving Anticoagulation
at home
• Weight based intermediate dose prophylactic UFH or LMWH
Anticoagulation
(e.g., Enoxaparin 0.5mg/kg per dose SC BD) ##
➢ Conventional dose prophylactic UFH or LMWH## (weight based e.g.,
➢ Tab Ivermectin (200 mcg/kg once a day enoxaparin 0.5mg/kg per day SC)
for 3 to 5 days) to be considered** Supportive measures
Monitoring • Maintain euvolemia (if available, use dynamic measures for
➢ Inhalational Budesonide (given via ➢ Clinical Monitoring: Work of breathing, Hemodynamic instability assessing fluid responsiveness)
MDI/DPI at a dose of 800 mcg BD for 5 to Change in oxygen requirement • If sepsis/septic shock: manage as per existing protocol and local
7 days) to be given if symptoms (fever antibiogram
and/or cough) are persistent beyond 5 ➢ Serial CXR; HRCT chest to be done ONLY If there is worsening Monitoring
days of disease onset# • Serial CXR; HRCT chest to be done ONLY if deteriorating
➢ Lab monitoring: CRP and D-dimer every 48 to 72 hrly; CBC KFT, LFT • Lab monitoring: CRP and D-dimer 24-48 hourly; CBC, KFT, LFT
every 24 to 48 hrly; IL-6 levels to be done if deteriorating (Subject to
daily; IL-6 to be done if deteriorating (subject to availability)
availability)
➢ Systemic Steroids NOT indicated
*High-risk for severe disease or mortality
✓ Age > 60 years
After clinical Improvement discharge
✓ Cardiovascular disease including hypertension and CAD as per revised discharge criteria
✓ DM (Diabetes mellitus) and other immunocompromised states
✓ Chronic lung/kidney/liver disease
EUA/Off label (use based on limited available evidence and only in specific circumstances):
✓ Cerebrovascular disease
✓ Obesity
➢ Remdesivir (EUA) may be considered ONLY in patients with
o Moderate to severe disease (i.e., requiring SUPPLEMENTAL OXYGEN), AND
# MDI – Metered dose inhaler; DPI – Dry powder inhaler o Who are within 10 days of symptom onset, with
**Use should be avoided in pregnant and lactating women o No renal or hepatic dysfunction (eGFR <30 ml/min/m2; AST/ALT >5 times ULN (Not an absolute contradiction), AND
o Recommended dose is 200 mg IV on day 1 f/b 100 mg IV OD for next 4 days
^^Higher chances of NIV failure ➢ Tocilizumab (Off-label) may be considered when ALL OF THE BELOW CRITERIA ARE MET
## LMWH: Low Molecular Weight Heparin: if no contraindication or high
o Presence of severe disease (Preferably within 24 to 48 hours of onset of severe disease/ICU admission)
risk of bleeding; UFH: Unfractionated heparin o Significantly raised inflammatory markers (CRP &/or IL-6)
o Not improving despite use of steroids
o No active bacterial/fungal/tubercular infection
o The recommended dose is 4 to 6 mg/kg (usually a dose of 400 mg in a typical 60kg adult) in 100 ml NS over 1 hour (single dose)
o
➢ Convalescent plasma (Off label) may be considered when following criteria are met
o Early moderate disease (Preferably within 7 days of symptom onset)
o Availability of high titre donor plasma (Signal to cutoff ratio (S/O) >3.5 or equivalent depending on the kit being used)
o Usual dose is 200 ml given over a period of 2 or more hours
Department of Medicine, AIIMS (ND)
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Veterinary Parasitology: Elizabeth B. Mitchell, John W. Mccall, S. Theodore Chester, Diane LarsenDocument3 pagesVeterinary Parasitology: Elizabeth B. Mitchell, John W. Mccall, S. Theodore Chester, Diane LarsenChorrinha ChorraNo ratings yet
- Provimi Product Catalogue PDFDocument116 pagesProvimi Product Catalogue PDFBINAY KUMAR YADAVNo ratings yet
- Cenforce 100mgDocument2 pagesCenforce 100mgjean greyNo ratings yet
- OBAnnual39 PDFDocument1,561 pagesOBAnnual39 PDFAlphamale AlphamaleNo ratings yet
- Student Project: Applications of Exponential Functions: Pure Mathematics 30Document8 pagesStudent Project: Applications of Exponential Functions: Pure Mathematics 30Pie PeyNo ratings yet
- Biopharmaceutics Classification System: Systematic Reviews in Pharmacy January 2010Document9 pagesBiopharmaceutics Classification System: Systematic Reviews in Pharmacy January 2010afdsafNo ratings yet
- Journal of Communication Disorders: Donna C. Thomas, Patricia Mccabe, Kirrie J. BallardDocument14 pagesJournal of Communication Disorders: Donna C. Thomas, Patricia Mccabe, Kirrie J. BallardFernanda FreitasNo ratings yet
- Vasopressors and Nutrition Therapy Safe Dose For T 210523 124532Document7 pagesVasopressors and Nutrition Therapy Safe Dose For T 210523 124532Natália GiovanaNo ratings yet
- Cleaning Validation MACO Swab Rinse OvaisDocument5 pagesCleaning Validation MACO Swab Rinse OvaisOvais08100% (9)
- Parkinson TreatmentDocument22 pagesParkinson TreatmentMuhammad HilmiNo ratings yet
- Therapeutics HandbookDocument49 pagesTherapeutics Handbookaasdf100% (1)
- Taking Medication HisotoriesDocument14 pagesTaking Medication HisotoriesCésar Augusto Sánchez SolisNo ratings yet
- Complex Protrombina Huvh 01 10Document11 pagesComplex Protrombina Huvh 01 10Santiago Rodriguez MoralesNo ratings yet
- T56 - Martindale The Complete Drug Reference 38th P 2114Document1 pageT56 - Martindale The Complete Drug Reference 38th P 2114Martiana CandraNo ratings yet
- KrishnamurthyDocument62 pagesKrishnamurthymihaipopescu0100% (1)
- Full Download Goulds Pathophysiology For The Health Professions 5th Edition Vanmeter Test BankDocument36 pagesFull Download Goulds Pathophysiology For The Health Professions 5th Edition Vanmeter Test Bankjamboolflexuous.el624100% (29)
- Ebook Physician Assistant A Guide To Clinical Practice PDF Full Chapter PDFDocument67 pagesEbook Physician Assistant A Guide To Clinical Practice PDF Full Chapter PDFdavid.dover659100% (29)
- Cold Water Extraction of OpioidsDocument36 pagesCold Water Extraction of OpioidsC BerteNo ratings yet
- Surgical Solutions For Gerbils Analgesia and Anaesthesia IdeasDocument6 pagesSurgical Solutions For Gerbils Analgesia and Anaesthesia IdeasCristian FloreaNo ratings yet
- See Full Prescribing Information For Complete Boxed WarningDocument31 pagesSee Full Prescribing Information For Complete Boxed Warningfelipee20No ratings yet
- PPAG Alcohol Content in Peds Meds Poster-4!10!18Document2 pagesPPAG Alcohol Content in Peds Meds Poster-4!10!18herlinaNo ratings yet
- Switchguide 160106 enDocument13 pagesSwitchguide 160106 enfevangelisti1No ratings yet
- Hospital Pharmacy NotesDocument15 pagesHospital Pharmacy NotesChunnie JakosalemNo ratings yet
- DPCODocument30 pagesDPCOArya SreedharanNo ratings yet
- HSCMDA GuidelinesDocument43 pagesHSCMDA GuidelinesMaisa Rose Bautista Vallesteros100% (1)
- CHUVADocument46 pagesCHUVAJUAN MIKHAELNo ratings yet
- Ramadan Resource PackDocument24 pagesRamadan Resource Packkraft10No ratings yet
- Problems: 5: VancomycinDocument4 pagesProblems: 5: VancomycinSaul RuizNo ratings yet
- Kalyanaka Ghrita ConstituentsDocument8 pagesKalyanaka Ghrita ConstituentsQadiir AlamNo ratings yet
- Ashtavargam Kashayam - Benefits, Dose, Side Effects, IngredientsDocument17 pagesAshtavargam Kashayam - Benefits, Dose, Side Effects, IngredientsRaju GovindNo ratings yet