Professional Documents
Culture Documents
Case Study Objective Pcs
Uploaded by
Amirul Adham0 ratings0% found this document useful (0 votes)
7 views2 pagesCopyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views2 pagesCase Study Objective Pcs
Uploaded by
Amirul AdhamCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Objective
1. To understand the key features of coordination compounds used in industry,
including its molecular structure, properties of the compound and mechanism involve
in particular industries.
2. To studies the name of coordination compounds that play an important roles in
industry.
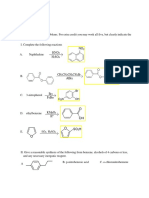
Phthalocyanine (Pc)
Figure 1. Molecular structure of the reactive dye turquoise blue 15 (AT15).
The name of the complex compound is Phthalocyanine (Pc), it also known as
Turquoise blue 15 (AT15). Phthalocyanines, a class of organic dyes, and today are the
main organic blue and green pigments in the industry. Phthalocyanine based colouring
materials are highly useful in developing blue and green hues, which are the most
stable organic colourants due to extensive structural resonance stabilisation. It is
resistant to almost all acids, alkalis, oxidising and reducing agents; planner and
tetraazo derivative of four isoindole units connected by four nitrogen atoms that
together form an internal 16-membered ring of alternate carbon and nitrogen atoms.
The blue hue is dull in some ways and a brilliant intenses hue with λmax 670–685 nm
is developed only when it reacts with a metal atom, preferably that with copper
known as copper phthalocyanine which contains three benzonoid and one p-quinonoid
outer ring. Phthalocyanines form complexes with a variety of chemical elements. The
most important phthalocyanine, copper phthalocyanine, is produced worldwide. It is
also a reactive dye widely used in the textile industry to color natural fibers. In
addition to their extensive use as pigments and dyes, this versatility gives rise to many
applications, such as catalysts, liquid crystals, electrochromic and photochromic
substances, data storage systems, photodynamic cancer therapy agents, photoactive
units, chemical sensors, and nonlinear optical devices. However, 90% of the
phthalocyanines are used as pigments for printing inks, paints, coatings, plastics,
textile, and spin dyeing industries. Due to environmental issues, the presence of these
dyes in effluent and industrial wastewater is of great concern. This is because, during
textile processing, inefficiencies in the dyeing process result in 10–15% of all dye
stuff being lost directly to wastewater which ultimately finds its way into the
environment. Phthalocyanine dyes are a kind of near-IR photosensitizer with
promising electrochemical, photochemical, and thermal properties. In order to
facilitate the dye-sensitization process, structural modification of these dyes should be
carried out to improve their solubility. The strong aggregation on the semiconductor
surface is a major problem for phthalocyanine dye. Usually, the co-adsorbent should
be added in the dye bath to suppress the dye aggregation and improve the photovoltaic
properties. A large number of phthalocyanine dyes have been developed and applied
in DSCs. Among these phthalocyanine dyes, zinc (Zn) phthalocyanines have been
investigated widely by scientists due to their low cost and suitable energy levels.
Reference
1. Taniyuki Furuyama, et al. 2013. “Design, Synthesis, and Properties of
Phthalocyanine Complexes with Main-Group Elements Showing Main Absorption
and Fluorescence beyond 1000 nm” Journal of the American Chemical Society
136, 2, 765–776.
2. Andreas Gouloumis, et al. 2000 “Synthesis and Electrochemical Properties of
Phthalocyanine–Fullerene Hybrids” Chemistry A European Journal. Volume 6, Issue
19, pages 3475-3653
3. Altuğ Mert Sevim, et al. 2011 “Preparation of heterogeneous phthalocyanine
catalysts by cotton fabric dyeing” Dyes and Pigments. Volume 89, Issue 2, Pages
162-168
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (589)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5806)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Biomolecule ChartDocument1 pageBiomolecule ChartJaylen GardnerNo ratings yet
- Aluminum CorrosionDocument1 pageAluminum CorrosionthanhnguyenhhvnNo ratings yet
- 7.P-Block Elements (Group 15 To 18) KCET PYQsDocument2 pages7.P-Block Elements (Group 15 To 18) KCET PYQsPunith kumarNo ratings yet
- Nitric Acid - Nitrous Acid - Nitrogen Oxides - Ullman's EncyclopediaDocument49 pagesNitric Acid - Nitrous Acid - Nitrogen Oxides - Ullman's Encyclopediapoly6icsNo ratings yet
- Business Plan Ent FinalizeDocument103 pagesBusiness Plan Ent FinalizeAmirul AdhamNo ratings yet
- ENT300 - Business Plan NattyPop PopcornDocument28 pagesENT300 - Business Plan NattyPop PopcornAmirul AdhamNo ratings yet
- The Family Tree of Stainless SteelDocument14 pagesThe Family Tree of Stainless Steelnike_y2kNo ratings yet
- Reflection Report FSGDocument8 pagesReflection Report FSGAmirul AdhamNo ratings yet
- FSG301 Case Study Group 4 RAS1205GDocument16 pagesFSG301 Case Study Group 4 RAS1205GAmirul AdhamNo ratings yet
- SPM Practice Chap3 F4Document7 pagesSPM Practice Chap3 F4Jonathan LingNo ratings yet
- Polymer IndustryDocument6 pagesPolymer IndustryAmirul AdhamNo ratings yet
- PHY351 - Written AssignmentDocument3 pagesPHY351 - Written AssignmentAmirul AdhamNo ratings yet
- Data Sheet Experiment 1 Predicting Molecular Shape and Polarity Using VSEPR TheoryDocument4 pagesData Sheet Experiment 1 Predicting Molecular Shape and Polarity Using VSEPR TheoryAmirul AdhamNo ratings yet
- CHM361 Case Study GroupDocument4 pagesCHM361 Case Study GroupAmirul AdhamNo ratings yet
- Proposal Furnihome SDN BHD FSGDocument10 pagesProposal Furnihome SDN BHD FSGAmirul AdhamNo ratings yet
- Case Study FSG301Document2 pagesCase Study FSG301Amirul AdhamNo ratings yet
- Pt. Atlantic Intraco - Safety Data Sheet: Calcium Oxide - CaoDocument5 pagesPt. Atlantic Intraco - Safety Data Sheet: Calcium Oxide - CaoAreIf Cron BmxStreetNo ratings yet
- Paper PDFDocument28 pagesPaper PDFudaysrinivasNo ratings yet
- Chemistry-Orgo II Exam 1 Version A (UD) Answer KeyDocument8 pagesChemistry-Orgo II Exam 1 Version A (UD) Answer KeyNesrine LaradjiNo ratings yet
- Cambridge International Advanced Subsidiary and Advanced LevelDocument12 pagesCambridge International Advanced Subsidiary and Advanced LevelMuhammad AhmedNo ratings yet
- Material Safety Data Sheet: Product Name: MOBILUX EP 2Document9 pagesMaterial Safety Data Sheet: Product Name: MOBILUX EP 2Sos contingencias sasNo ratings yet
- Role of Hexavalent Chromium in The Inhibition of Corrosion of Aluminum AlloysDocument9 pagesRole of Hexavalent Chromium in The Inhibition of Corrosion of Aluminum AlloysanissaNo ratings yet
- Experiment 2Document8 pagesExperiment 2Alok VermaNo ratings yet
- ICSE MCQ IonicCompoundsDocument2 pagesICSE MCQ IonicCompoundsSantanuNo ratings yet
- Instruction Manual Alfacond enDocument22 pagesInstruction Manual Alfacond enAjayNo ratings yet
- Fundamentals of Ecology Eugene P Odum SaDocument1 pageFundamentals of Ecology Eugene P Odum SaabdulNo ratings yet
- General Micro Lab 5 Biochemical Testing - Fall 2021Document14 pagesGeneral Micro Lab 5 Biochemical Testing - Fall 2021julioNo ratings yet
- 9701 w04 QP 2Document12 pages9701 w04 QP 2Hubbak KhanNo ratings yet
- Lindsay Et Al, 1959 IIDocument5 pagesLindsay Et Al, 1959 IIexportar2299No ratings yet
- IUPAC NOMENCLATURE by Bharat PanchalDocument34 pagesIUPAC NOMENCLATURE by Bharat Panchalvansh ranaNo ratings yet
- Alkanes - Alkenes - Alkynes - DPP 2Document3 pagesAlkanes - Alkenes - Alkynes - DPP 2Vishal_93No ratings yet
- List of Persistent and Non Persistent CargoDocument33 pagesList of Persistent and Non Persistent CargoJeet Singh100% (1)
- Banned CMR enDocument10 pagesBanned CMR enhavisha_24No ratings yet
- Chemistry - ElementsDocument2 pagesChemistry - ElementsJasmine Laprades100% (1)
- Charles John S. Garcia BSAG-1101 ANSCI: Identification: Answer and Review The FollowingDocument6 pagesCharles John S. Garcia BSAG-1101 ANSCI: Identification: Answer and Review The FollowingMelvin Pogi138No ratings yet
- Lab Report PDFDocument2 pagesLab Report PDFAl-Vi John CorpuzNo ratings yet
- Bing MM 347 Presentacion SynthesiaDocument18 pagesBing MM 347 Presentacion SynthesiaA MahmoodNo ratings yet
- Aldehydes, Ketons and Carboxylic Acid Unit 11 MCQDocument4 pagesAldehydes, Ketons and Carboxylic Acid Unit 11 MCQAbhinav TripathiNo ratings yet
- Lecture 11 Hydrogenation and Its ThermodynamicsDocument28 pagesLecture 11 Hydrogenation and Its ThermodynamicsHuraira AbidNo ratings yet
- Tesis Extraction, Identification and Antioxidant Activity of The Phenolic Secondary Metabolites Isolated From The Leaves, Stems and Fruits of Two Shrubs of The Ericaceae FamilyDocument259 pagesTesis Extraction, Identification and Antioxidant Activity of The Phenolic Secondary Metabolites Isolated From The Leaves, Stems and Fruits of Two Shrubs of The Ericaceae FamilylilisacasNo ratings yet