Professional Documents
Culture Documents
ECG & Pulse Protocol
Uploaded by
uracan 322Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ECG & Pulse Protocol
Uploaded by
uracan 322Copyright:
Available Formats
Student Protocol
Electrocardiogram & Peripheral Circulation
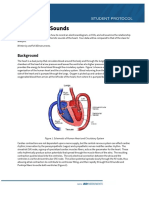
The heart is a dual pump that pushes blood around the body and through the lungs. The beating of
the heart results in a blood flow that is itself rhythmic. In this lab you will measure the flow of blood
through the finger of a student volunteer and correlate it with the ECG. In addition you will palpate
various arteries, and look at peripheral circulation.
Written by staff of ADInstruments.
Background
The arterial system functions as a pressure reservoir. Blood leaves the arterial system continuously through the
capillaries, but enters intermittently from the heart. The ventricles contract during systole; the semilunar valves
open and blood flows into the arterial system. At this point the arteries expand and the blood pressure
increases. Systolic pressure is the peak pressure value. The relaxation of the ventricles is called diastole. During
diastole, the ventricles fill with blood from the atria and veins, and prepare for the next systole. Simultaneously,
blood flows out of the arterial system through the capillaries and the arterial pressure decreases. When the
arterial blood pressure is at its lowest — immediately before the contracting ventricle pushes blood into the
arteries — this value is called the diastolic pressure. Although the variation in arterial blood pressure during the
cardiac cycle is smoothed out by the inherent elasticity of the major arteries, blood still exhibits pulsatile flow
through the arteries and arterioles.
Required Equipment
A computer system
Chart software
PowerLab (with built-in Bio Amp or PowerLab and Bio Amp front-end)
Five-lead Shielded Bio Amp Cable, & snap-connect Lead Wires
Reusable clamp electrodes or disposable adhesive electrodes
Electrode cream (for use with the clamp electrodes)
Alcohol swabs (70% ethanol on cotton wool or paper tissue); optional
Ballpoint pen
Abrasive pads/gel
Finger Pulse Transducer
Procedures
Set up and equipment calibration
The following setup is used for Exercise 1; the ECG connections are then removed for the subsequent exercises,
which only require the Finger Pulse Transducer (Figure 1).
SPB09e Page 1 of 14 7 December 2005
Student Protocol
Figure 1. Equipment setup for this experiment showing the finger pulse and Bio Amp connections.
Subject preparation
1. With the PowerLab turned off, plug the Bio Amp cable into the Bio Amp.
2. Instruct the volunteer for the experiment to remove any watches and/or jewelry from their wrists and ankles.
3. Connect the lead wires to Earth, CH1 negative, and CH1 positive on the Bio Amp cable.
4. If alcohol swabs are available, firmly swab the skin with them in each area where electrodes will be placed
(Figure 2). Using a pen, mark each area with a small cross. Lightly abrade the skin at these areas with an
abrasive pad/gel. This decreases the electrical resistance of the outer layer of skin and ensures good
electrical contact.
5. If using the reusable clamp electrodes, apply a small amount of electrode cream to the electrodes, attach the
electrodes to the subject as described below, and connect the electrodes to the leads. If using the
disposable electrodes (which have electrode gel on them already), just attach the electrodes to the subject
as described below, and connect the electrodes to the leads.
6. Ensure the volunteer is relaxed and sits as still as possible to minimize any signal artifacts from movement.
SPB09e Page 2 of 14 7 December 2005
Student Protocol
Attaching the electrodes
Standard connection: attach the positive electrode to the left wrist, the negative to the right wrist, and the
ground to the right leg. If after looking at the signal in the Bio Amplifier dialog window during the first exercise
this does not produce a good signal, try using the alternative method shown in Figure 2.
Alternative connection: attach the positive electrode to the left upper arm, the negative to the right upper
arm and the ground to either wrist (Figure 2). NOTE: Do not place the electrodes over the major muscles
of the upper arm because muscle activity interferes with the signal recorded from the heart. Attach
the electrodes on the outer side of the arm, midway between the elbow and the shoulder.
Figure 2. Connecting the electrodes to the volunteer: two alternative methods.
Software calibration
To set up recording for this experiment, load a settings file from the Experiments Gallery.
1. Turn on the PowerLab.
2. Launch Chart from the computer. If the Experiments Gallery dialog window does not appear in front of the
Chart window, choose the Experiments Gallery… command from the File menu.
3. In the Experiments Gallery dialog window, select this experiment (Electrocardiogram & Peripheral Circulation)
in the left-hand list. Select the ECG and Pulse Settings file in the right-hand list then click the Open button to
apply those settings.
4. After a short time, the Chart window on the computer screen should be set up for the experiment. Channel 1
labeled ‘Vol. Pulse’, Channel 2 ‘Blood Flow’ and Channel 3 ‘ECG’.
Note: Channel 2 (Blood Flow) is the raw signal from the finger pulse transducer and is an indication of the net
rate of blood flow into the finger pulp. The time integral of Channel 2 is displayed on Channel 1, and gives an
idea of the change in finger pulp volume over time.
SPB09e Page 3 of 14 7 December 2005
Student Protocol
5. In Chart, choose the Bio Amplifier… item from the Channel 3 (ECG) Channel Function pop-up menu. Observe
the signal.
NOTE: If the ECG cannot be seen, check that all three electrodes are correctly attached. Adjust the range if
necessary. If the signal is noisy and indistinct, make sure that the volunteer is relaxed; consider using the
alternative attachment positions shown in Figure 2.
6. Click the OK button to return to the Chart window.
7. Choose the Input Amplifier… item from the Channel 2 (Blood Flow) Channel Function pop-up menu. Adjust
the value in the Range drop-down list of the dialog window that appears so that the signal occupies about a
half to two thirds of full scale when the volunteer has both hands in their lap.
8. Click the OK button to return to the Chart window.
Exercise 1: ECG and volume pulse at rest
Objectives
To measure the ECG and volume pulse in a resting volunteer, and analyze and correlate the resultant signals.
Procedure
1. Click the Start button, and record for ten seconds. During this time, add a comment to the data file with
the subject’s name.
2. Click the Stop button. The waveforms should look something like those in Figure 3.
3. If instructed to do so, repeat steps 1 and 2 for the other members of the group.
4. Save the data by choosing “Save as…” from the File menu in Chart.
Dicrotic notch
Waveform Cursor
Marker
Figure 3. The type of signals you should see in Exercise 1.
SPB09e Page 4 of 14 7 December 2005
Student Protocol
Exercise 2: The volume pulse
Objectives
To measure the volume pulse in a series of resting volunteers, analyze the resultant signals, and look at the
variations.
Procedure
1. Remove the ECG equipment from the student volunteer.
2. From the File menu or Experiments Gallery, open the file “Volume Pulse Settings”.
3. Click Start to begin recording. If necessary, adjust the range for Channel 2 (Blood Flow) using the Range
drop-down list.
4. Record ten seconds of pulse data. Add a comment to the recording with the volunteer’s name.
5. Click Stop.
6. Transfer the finger pulse transducer to another student, and repeat steps 3-5.
Note: Channel 1 (Vol. Pulse) displays a computation (the time integral) based on data in Channel 2, so changing
acquisition parameters in Channel 2 will also affect Channel 1.
Exercise 3: Palpation of arterial pulses
Objectives
Palpate the radial, ulnar, brachial, carotid and facial pulses, and attempt to understand what is involved in the
pulse.
Procedure
Brachial artery Radial artery
Ulnar artery
Figure 4. Sites for palpation of the radial, ulnar and brachial pulses in the left arm.
1. Feel the subject’s radial pulse, at the place indicated in Figure 4. To do this use the first three fingers, index,
middle and ring fingers, placed in a line along the length of the radial artery.
SPB09e Page 5 of 14 7 December 2005
Student Protocol
Note: Don’t use the thumb for palpation — it has a strong pulse in it, and you may end up feeling your own
pulse instead of the subject’s. Also, don’t press too hard — only light to moderate pressure is needed to feel a
pulse.
2. Attempt to feel the subject’s ulnar pulse, at the place indicated in Figure 4. No pulse can be felt in most
people.
3. Feel the brachial artery pulse at the elbow, at the place indicated in Figure 4. When looking for the arteries,
it is best to glide the fingers back and forth slowly over the region rather than immediately applying pressure.
4. Feel the carotid pulse in the neck, by placing the index, middle and ring fingers in a line and pressing gently
to one side of the trachea. The carotid pulse is very strong and easily palpated.
5. Feel the facial pulse. It is a delicate pulse requiring skill to discern. The facial artery passes from the neck
towards the face, by winding around the edge of the mandible about 1–2 cm in front of the angle.
a) Locate the angle of the mandible.
b) Feel the horizontal edge of the mandible in front of the angle.
c) Use the ball of the index finger to palpate very gently 1–2 cm in front of the angle.
6. Doctors are trained to assess various aspects of a pulse: the rate, rhythm, amplitude and quality. (For
example the rate might be 72 beats per minute, rhythm regular or irregular, amplitude full and quality
“thready” or collapsing.) Which of these parameters do you think would be easy to assess, and which would
be difficult?
Exercise 4: Arterial anastomoses in the hand
Objectives
Show that the arterial blood supply to the fingers derives from both radial and ulnar arteries by way of
anastomoses (connections between the vessels) in the hand.
Procedure
1. Place the finger pulse transducer on the distal segment of the middle finger of the student volunteer (Figure
1).
2. Click the Start button to start recording, and adjust the range if necessary.
3. Apply firm pressure with the ball of the thumb over the brachial artery (Figure 4), and observe the change, if
any, in the amplitude of the pulse signal. Apply pressure for 5–10 seconds only, and then release it. Add
the comment “brachial” to the recording when starting to apply pressure. Add the comment “release”
when the pressure is released.
4. Apply firm pressure over the radial artery (Figure 4), and observe the change (if any) in the amplitude of the
pulse signal. Release the pressure after 5–10 seconds. Add the comment “radial” when beginning to apply
pressure. Add the comment “release” when the pressure is released.
5. Apply firm pressure over the ulnar artery (Figure 4), and observe the change (if any) in the amplitude of the
pulse signal. Release the pressure after 5–10 seconds. Add the comment “ulnar” when beginning to apply
pressure. Add the comment “release” when the pressure is released.
SPB09e Page 6 of 14 7 December 2005
Student Protocol
6. Apply firm pressure over both the radial and ulnar arteries simultaneously, and observe the change (if any) in
the amplitude of the pulse signal. Release the pressure after 5–10 seconds. Add the comment “radial and
ulnar” when beginning to apply pressure. Add the comment “release” when the pressure is released.
7. Click the Stop button and observe the data trace. The waveforms should look something like those in Figure
5.
Figure 5. Typical results from Exercise 4. Adjust the View buttons to expand
or compress the data view.
Exercise 5: The effect of cold on volume pulse
Objectives
Examine the effect of cold on the amplitude of the finger pulse.
1. Remove the finger pulse transducer from the hand.
2. Immerse the hand and forearm in a basin of ice water.
3. Re-attach the finger pulse transducer.
4. Click Start and begin recording.
5. Record the slow changes in flow during recovery as the hand warms up over several minutes. After five
minutes, click Stop.
6. Use the View buttons in the Chart window to compress the view horizontally (to about 50:1 or 100:1) to see
slow trends over time.
SPB09e Page 7 of 14 7 December 2005
Student Protocol
Analysis
Exercise 1: ECG and volume pulse at rest
1. Drag the Marker to the peak of a QRS complex in Channel 3.
2. Observe the Waveform Cursor in Channel 2 (Blood Flow). Move the cursor to the right so that it lies on
the peak of the blood flow trace that follows the QRS complex (Figure 3).
3. Note the time difference, ∆t, from the Rate/Time display at the top right of the Chart window. This is the
time between the two events. Record this value in Table 1 of the Data Notebook.
4. If recordings from more than one member of the group have been made, record those data into Table 1 of
the Data Notebook.
Exercise 2: The volume pulse
Select a block of data containing ten pulse beats.
Click the Zoom window toolbar button to enter the Zoom view.
Using the Marker and Waveform Cursor, determine the pulse amplitude, beat duration and heart rate for each
member of the group and record these data in Table 2 of the Data Notebook.
To measure pulse amplitude:
1. Place the Marker tool on the baseline of the pulse waveform.
2. Drag the Waveform Cursor to the maximum peak value.
3. Record the value for amplitude in millivolts; displayed at the top of the Zoom window.
To measure beat duration:
1. Place the Marker tool on the baseline of the waveform just before a pulse beat.
2. Drag the Waveform Cursor over the trace until it reaches the end of the pulse beat.
3. Record the t value displayed in the upper part of the Zoom window.
To calculate heart rate:
1. Place the Marker tool on a pulse beat peak.
2. Drag the Waveform Cursor to the peak of the last pulse beat in your selection.
3. Record the t from the Zoom window display.
4. Use the following equation to determine pulse rate:
t
HR 60
n 1
Where n= the total number of beats used to determine t and time is in seconds.
SPB09e Page 8 of 14 7 December 2005
Student Protocol
Exercise 4: Arterial anastomoses in the hand
1. Select a block of data from your trace containing the volume pulse recording during brachial artery
compression.
2. Click the Zoom window toolbar button to enter the Zoom view.
3. Place the Marker on the baseline of the waveform (between pulse beats).
4. Drag the Waveform Cursor to the maximum of a pulse beat and record the amplitude value in the Data
Notebook, Table 3.
5. Express the volume pulse amplitude as the percent of “normal” values for each vessel by using the following
equation:
A(comp)
100%
A(norm)
Where A (comp) is the maximum amplitude of the volume pulse when an artery was compressed and A (norm) is the maximum
amplitude of the volume pulse when no arteries were compressed.
6. Repeat steps 1-5 for the other arteries compressed.
Exercise 5: The effect of cold on volume pulse
Using the Marker and Waveform Cursor, record the pulse amplitude of a representative volume pulse beat
every thirty seconds over the duration of the recording for Exercise 5. Record these values in Table 4 of the
Data Notebook.
SPB09e Page 9 of 14 7 December 2005
Student Protocol
Data Notebook
Table 1. The relationship between ECG and the volume pulse
Group member name t between QRS and volume pulse (sec)
Table 2. Properties of the volume pulse
Group member name Pulse amplitude (mV) Pulse duration (sec) Heart rate (BPM)
Table 3. Arterial anastomoses in the hand
Relative pulse amplitude (% of normal value)
Radial and Ulnar
Brachial artery Radial artery Ulnar artery
arteries
SPB09e Page 10 of 14 7 December 2005
Student Protocol
Table 4. The effect of cold on volume pulse
Time since Volume pulse
immersion (seconds) amplitude (mV)
SPB09e Page 11 of 14 7 December 2005
Student Protocol
Study Questions
Exercise 1
1. What produces the QRS complex in the ECG?
2. What does the peak in the blood flow trace represent?
3. Which processes take place between these two events?
4. Does the falling phase of the volume pulse have a small, transient plateau or upward deflection, as shown
in the upper trace of Figure 3 ? This is called a dicrotic notch.
5. Would you expect a transient increase in blood pressure as the elastic arteries recoil after being stretched
by blood entering from the ventricles? Explain your reasoning.
SPB09e Page 12 of 14 7 December 2005
Student Protocol
Exercise 2
1. Do all traces have a dicrotic notch?
2. Is there any obvious correlation between the size of the dicrotic notch and an individual’s age or apparent
fitness?
Exercise 3
1. When you feel a pulse, do you feel (a) the blood flow, (b) the pressure wave, or (c) brief changes in
diameter of the artery due to the pressure wave?
2. Anatomical sites where a pulse can be palpated often correspond to ‘pressure points’ for stopping
hemorrhage in first-aid treatment. Why?
3. Why is it usually difficult to feel the ulnar pulse?
Exercise 4
1. Why did the pulse disappear when the brachial artery was compressed?
SPB09e Page 13 of 14 7 December 2005
Student Protocol
2. Did the pulse disappear completely when the radial or ulnar artery alone was compressed? If not, why
not?
3. There is much anatomical variation from person to person, but in most people, blood flow to the fingers is
derived mainly from the ulnar artery, with a smaller contribution from the radial artery. On the basis of
your results, does the subject seem to conform to the general rule?
4. What is the function of arterial anastomoses?
Exercise 5
1. Speculate on the adaptive significance of a reduction in peripheral blood flow in cold conditions.
2. How is the reduction in peripheral blood flow achieved?
Copyright © 2005 ADInstruments. All rights reserved.
MacLab and PowerLab are registered trademarks, and Chart and Scope are trademarks, of ADInstruments. Windows and the Windows
logo are either trademarks or registered trademarks of Microsoft Corporation. Macintosh and the Mac logo are either trademark s or
registered trademarks of Apple Computer, Inc. Other trademarks are the properties of their respective owners.
www.ADInstruments.com
SPB09e Page 14 of 14 7 December 2005
You might also like
- Electrocardiography - 2Document17 pagesElectrocardiography - 2salochinNo ratings yet
- The Little Black Book of Ecg Secrets PDFDocument12 pagesThe Little Black Book of Ecg Secrets PDFamaandreiNo ratings yet
- PowerLab ECGDocument11 pagesPowerLab ECGVaibhav Goyal50% (2)
- Emg ProtocolDocument14 pagesEmg ProtocolBrian ThompsonNo ratings yet
- EMG Recording of Voluntary and Evoked Muscle ActivityDocument7 pagesEMG Recording of Voluntary and Evoked Muscle ActivitysariNo ratings yet
- EMG ProtocolDocument11 pagesEMG ProtocolWayo Asakura100% (2)
- Lab 4 Handout Cardiac Physiology II (ECG BP HR)Document6 pagesLab 4 Handout Cardiac Physiology II (ECG BP HR)Wilson CheungNo ratings yet
- Blood Pressure: Experiment 3Document8 pagesBlood Pressure: Experiment 3Mohamed Abdenour ChettouhNo ratings yet
- ECG & Heart Sounds: Rest vs. ExerciseDocument13 pagesECG & Heart Sounds: Rest vs. ExerciseAnkur AthuniNo ratings yet
- Student ProtocolDocument14 pagesStudent ProtocolCristian AmadoNo ratings yet
- Lab 3Document10 pagesLab 3aamirkhan8632No ratings yet
- EMG Study ReviewDocument4 pagesEMG Study ReviewShauki AliNo ratings yet
- ECG Lab: Heart Rate & Arterial FunctionDocument13 pagesECG Lab: Heart Rate & Arterial FunctionAli AliNo ratings yet
- Frog Heart ProtocolDocument9 pagesFrog Heart ProtocolMatthew SANo ratings yet
- 55) EEG and EMGDocument12 pages55) EEG and EMGShaibal BaruaNo ratings yet
- Introduction To Psychophysiology Student ProtocolDocument8 pagesIntroduction To Psychophysiology Student ProtocolpiaNo ratings yet
- 4 2 3 A Ekg Student Response Sheet (Revised 2 23 15)Document8 pages4 2 3 A Ekg Student Response Sheet (Revised 2 23 15)api-281824634100% (1)
- EMG MuscleStrength LS2 ELVISDocument15 pagesEMG MuscleStrength LS2 ELVISlabsoneducationNo ratings yet
- Ecg Lab Bio350Document13 pagesEcg Lab Bio350Robin Lewis CooperNo ratings yet
- FrogHeart Physiosp05Document12 pagesFrogHeart Physiosp05Frannie NillamaNo ratings yet
- Experiment 1 ElectrocardiographyDocument6 pagesExperiment 1 ElectrocardiographyMahdi BraitehNo ratings yet
- CardiovascularDocument16 pagesCardiovascularMilena VargasNo ratings yet
- Report ECGDocument11 pagesReport ECGQuang Khưu Đoàn ĐứcNo ratings yet
- Lab05.Pulse OximeterDocument3 pagesLab05.Pulse OximeterulgenyNo ratings yet
- Fix Lab Report 3 (Content) PDFDocument12 pagesFix Lab Report 3 (Content) PDFLuh RikaNo ratings yet
- Analyze Heart Activity with EKGDocument7 pagesAnalyze Heart Activity with EKGshamroz_aslam7959No ratings yet
- Frog Heart Physiology: I. PurposeDocument15 pagesFrog Heart Physiology: I. PurposeMd. Ismail HosenNo ratings yet
- Measuring Blood Pressure LabDocument4 pagesMeasuring Blood Pressure Labhord72No ratings yet
- Instructions Set - FinalDocument6 pagesInstructions Set - FinalMaggie RoseNo ratings yet
- EKG Rest Versus ExerciseDocument9 pagesEKG Rest Versus Exercisedesiree2391744No ratings yet
- Simulator Model For Abnormal Pulse RateDocument4 pagesSimulator Model For Abnormal Pulse RatemuthukumartharaniNo ratings yet
- 418rle m2 Sl1+Basic+EcgDocument8 pages418rle m2 Sl1+Basic+EcgPaul SahagunNo ratings yet
- 418RLE M2 SL1 Basic ECGDocument8 pages418RLE M2 SL1 Basic ECGCamille Neypes CarreraNo ratings yet
- Lab 1Document4 pagesLab 1Han hoNo ratings yet
- Design_and_Build_ECG_SimulatorDocument4 pagesDesign_and_Build_ECG_SimulatorJaderson RobertoNo ratings yet
- Frog Nerve Lab Demonstrates Nerve PhysiologyDocument13 pagesFrog Nerve Lab Demonstrates Nerve PhysiologyAlyanna BlanciaNo ratings yet
- ECG2Document2 pagesECG2Pollen Siega BunalNo ratings yet
- Measuring Basic Observations Vital Signs OSCE GuideDocument9 pagesMeasuring Basic Observations Vital Signs OSCE GuidedrpeterimojeNo ratings yet
- Tutorial: EcgsimDocument6 pagesTutorial: EcgsimGustavo GuzmanNo ratings yet
- ECG Guide: Electrodes, Leads, ProcedureDocument4 pagesECG Guide: Electrodes, Leads, ProcedureDhanNie CenitaNo ratings yet
- Student ProtocolDocument14 pagesStudent ProtocolLaura Camila Bermeo PeraltaNo ratings yet
- School of Engineering Department of Biomedical Engineering: EENG424L-Medical Instrumentation I LabDocument8 pagesSchool of Engineering Department of Biomedical Engineering: EENG424L-Medical Instrumentation I LabMahdi BraitehNo ratings yet
- Blood Pressure and Exercise LabDocument6 pagesBlood Pressure and Exercise Labshanea bucknorNo ratings yet
- Electronics Digital StethoscopeDocument4 pagesElectronics Digital StethoscopeoyunpurevoyunaaNo ratings yet
- Exp 05Document4 pagesExp 05mohammeda AlmaitahNo ratings yet
- Electrocardiography Is The Most Commonly Used Test For EvaluatingDocument5 pagesElectrocardiography Is The Most Commonly Used Test For Evaluatingmyk_1102No ratings yet
- L14 ProcedureDocument13 pagesL14 ProcedureyanfuNo ratings yet
- Activity 2.2.3: It's All in The ReflexesDocument8 pagesActivity 2.2.3: It's All in The ReflexesAva PeloquinNo ratings yet
- Lab01 2019e151Document7 pagesLab01 2019e151Vegashkar 152No ratings yet
- Lab Manual LocomotorDocument18 pagesLab Manual LocomotorJetGoliath 26No ratings yet
- DTE Final NotesDocument141 pagesDTE Final NotesSajanNo ratings yet
- Lab Exercise 2 The Physiology of Skeletal Muscle: SafetyDocument11 pagesLab Exercise 2 The Physiology of Skeletal Muscle: SafetyNashitah AlwazNo ratings yet
- Heart Rate1 PDFDocument8 pagesHeart Rate1 PDFCerin NinanNo ratings yet
- Nabl 126Document19 pagesNabl 126ashishtrueNo ratings yet
- Practical-Guidelines-Electrotherapy Application DefDocument10 pagesPractical-Guidelines-Electrotherapy Application DefZahid HussainNo ratings yet
- Human Factors Engineering IE 442: Laboratory ManualDocument5 pagesHuman Factors Engineering IE 442: Laboratory Manualoscar cienciaNo ratings yet
- Electrocardiography (1) FinishDocument37 pagesElectrocardiography (1) FinishSangeeta Indoi100% (1)
- Lab7 InstructionDocument9 pagesLab7 InstructionHan hoNo ratings yet
- Lab ManualDocument36 pagesLab Manualrokini bioNo ratings yet
- physiology practical tasksDocument13 pagesphysiology practical tasksppdzssx7ghNo ratings yet
- ECG/EKG Interpretation: An Easy Approach to Read a 12-Lead ECG and How to Diagnose and Treat ArrhythmiasFrom EverandECG/EKG Interpretation: An Easy Approach to Read a 12-Lead ECG and How to Diagnose and Treat ArrhythmiasRating: 5 out of 5 stars5/5 (2)
- 2607 FullDocument94 pages2607 FullanindiawNo ratings yet
- Cardiac CatheterizationDocument25 pagesCardiac CatheterizationQueeny Anne ApilNo ratings yet
- Notes On Cardiovascular SystemDocument3 pagesNotes On Cardiovascular Systemmohamed waleedNo ratings yet
- Riphah International University: Cardiopulmonary Physical TherapyDocument6 pagesRiphah International University: Cardiopulmonary Physical Therapykhalid.zainabNo ratings yet
- CBSE Class 7 Science Worksheet (7) - 0Document4 pagesCBSE Class 7 Science Worksheet (7) - 0Yatish TiwariNo ratings yet
- DLL First Grading Period For Grade 9Document7 pagesDLL First Grading Period For Grade 9Melanie Tagudin TrinidadNo ratings yet
- Heart Disease Complicating PregnancyDocument169 pagesHeart Disease Complicating PregnancyRajeev Sood100% (2)
- Chapter 3 QuestionDocument9 pagesChapter 3 QuestionShila KaurNo ratings yet
- Nose To Brain: A Versatile Mode of Drug Delivery System: January 2017Document24 pagesNose To Brain: A Versatile Mode of Drug Delivery System: January 2017Jesi MawarniNo ratings yet
- Complications in Third and Fourth Stage of LaborDocument35 pagesComplications in Third and Fourth Stage of LaborHui Chen100% (2)
- All Type Essays - Biology emDocument27 pagesAll Type Essays - Biology emjoker boy100% (1)
- SOMATOM Volume Zoom Special VA40Document88 pagesSOMATOM Volume Zoom Special VA40shedear25No ratings yet
- Ventricular Arrythmias: Junctional TachycardiaDocument1 pageVentricular Arrythmias: Junctional TachycardiaLwin Maung Maung ThikeNo ratings yet
- Emergency Department STEMI Algorithm GuideDocument1 pageEmergency Department STEMI Algorithm GuideOgizWaraNo ratings yet
- Cardiovascular Physiology - Achilles Pappano, 10EDocument305 pagesCardiovascular Physiology - Achilles Pappano, 10EPanda Mimi100% (1)
- Valvular Heart DiseaseDocument32 pagesValvular Heart DiseasefallenczarNo ratings yet
- Body Fluids and CirculationDocument5 pagesBody Fluids and CirculationSheehan MathurNo ratings yet
- RAD RLE MCN 9 Case StudyDocument9 pagesRAD RLE MCN 9 Case StudyCathleen Nasis ForrosueloNo ratings yet
- Elsevier Adaptive Quizzing - Quiz Performance ULTIMODocument18 pagesElsevier Adaptive Quizzing - Quiz Performance ULTIMOInteresante interNo ratings yet
- Nursing Care Plan Impaired Skin Integrity Traction)Document2 pagesNursing Care Plan Impaired Skin Integrity Traction)deric100% (18)
- Form 2 Term 1 Schemes-2021Document8 pagesForm 2 Term 1 Schemes-2021Terence100% (1)
- Sensing Heart Beat and Body Temperature DigitallyDocument4 pagesSensing Heart Beat and Body Temperature DigitallyHASSAN RUSINo ratings yet
- Ecg TestDocument67 pagesEcg TestEm KayNo ratings yet
- CIE IGCSE BiologyDocument25 pagesCIE IGCSE Biologytgdzbspikio.comNo ratings yet
- Ipertensione Arteriosa PolmonareDocument33 pagesIpertensione Arteriosa PolmonareCarlo MaxiaNo ratings yet
- Coronary Heart DiseaseDocument25 pagesCoronary Heart DiseaseDr. Pardeep KumarNo ratings yet
- IGCSE Biology Transport in Animals NotesDocument62 pagesIGCSE Biology Transport in Animals NotesSir AhmedNo ratings yet
- Acyanitic DefectsDocument9 pagesAcyanitic DefectsHalla BennaaNo ratings yet
- 1 Fetal CirculationDocument21 pages1 Fetal Circulationdr_mohanad100% (1)