Professional Documents
Culture Documents
Oligodendroglioma in The Cervical Spinal Cord of A Dog: T. M, A. M - L, M. H - T, W. B
Uploaded by
Samir BazanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Oligodendroglioma in The Cervical Spinal Cord of A Dog: T. M, A. M - L, M. H - T, W. B
Uploaded by
Samir BazanCopyright:
Available Formats
524 Brief Communications and Case Reports Vet Pathol 41:5, 2004
Vet Pathol 41:524–526 (2004)
Oligodendroglioma in the Cervical Spinal Cord of a Dog
T. MAMOM, A. MEYER-LINDENBERG, M. HEWICKER-TRAUTWEIN, AND W. BAUMGÄRTNER
Abstract. A 7-year-old male castrated Yorkshire Terrier dog developed slowly progressive neurologic dis-
turbances consisting of difficulties in moving the neck, lack of proprioception, and tetraparesis 4 months prior
its death. Neurologic examination, computer tomography, and myelography resulted in the tentative diagnosis
of intramedullary cervicothoracic spinal cord lesion. At necropsy, an intramedullary cervical spinal cord mass
between C5 and C6 was noticed. Histologically, cells of this well-demarcated, nonencapsulated neoplasm were
arranged in sheaths or cords separated by a fine fibrovascular stroma. The polygonal to round tumor cells were
characterized by moderate pale, basophilic, and vacuolar cytoplasm and round to slightly oval, centrally located
nuclei with fine-stippled heterochromatin, a single nucleolus, and a very low mitotic activity. Tumor cells lacked
glial fibrillary acidic protein, vimentin, factor VIII–related, and cytokeratin antigen expression. Histologic and
immunohistochemical findings led to the diagnosis of a cervical spinal cord oligodendroglioma.
Key words: Dogs; immunohistochemistry; oligodendroglioma; spinal cord; tumor.
Intramedullary spinal cord tumors originating from neuro- ependymomas, and oligodendroglioma.4,5 Clinical signs are
ectodermal cells have been described only rarely.1,2,4,5,9 Spinal mostly due to the space-occupying effect of the tumor.4
cord tumors in dogs have been classified according to their This report describes the histologic, immunohistochemi-
location into three categories: extradural, intradural-extramed- cal, and clinical findings of a spinal cord mass of a 7-year-
ullary, and intramedullary tumors.1,3,6 Most canine spinal cord old male castrated Yorkshire Terrier. The animal had a 4-
tumors are found extradurally, whereas primary intradural tu- month history of increasing inability to move the neck. The
mors are less common.1,3,9 In a recent study of 33 spinal cord dog’s condition deteriorated despite supportive treatment,
tumors in dogs it was reported that five cases (15%) were clas- and the animal showed circling and paresis of the forelimbs.
sified as intramedullary tumors, such as two nephroblastomas, The dog was submitted for a diagnostic work-up to the Clin-
a glioma, an astrocytoma, and a metastasis of a carcinoma.1,2 ic for Small Animals, School of Veterinary Medicine in Han-
Intramedullary neuroectodermal tumors include astrocytomas, over, Germany. Neurologically, the animal displayed mildly
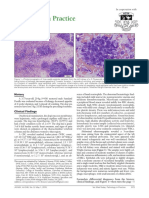
Fig. 1. Cervical spinal cord, canine oligodendroglioma. Well-demarcated intramedullary tumor (T) and compression
atrophy of the adjacent cervical spinal cord tissue between C5 and C6. Fig. 2. Spinal cord, canine oligodendroglioma.
Tumor cell proliferation and glomerular-like vascular proliferation at the periphery and reactive astrocytic response and
necrosis. N ⫽ necrosis, A ⫽ reactive astrocytic response (gemistocytes), T ⫽ tumor cells, arrow heads ⫽ characteristic
glomerular-like tufts. HE staining. Bar ⫽ 110 m.
Downloaded from vet.sagepub.com at ORILLIA CAMPUS LRC on March 18, 2015
Vet Pathol 41:5, 2004 Brief Communications and Case Reports 525
Fig. 3. Spinal cord, canine oligodendroglioma. Characteristic ‘‘honeycomb’’ cell pattern of an oligodendroglioma. HE
stain, Bar ⫽ 30 m. Fig. 4. Spinal cord, canine oligodendroglioma. Area of gemistocytic astroglial differentiation and
necrosis. A ⫽ reactive astrocytic response (gemistocytes), N ⫽ necrotic area. HE stain. Bar ⫽ 30 m. Fig. 5. Spinal cord,
canine oligodendroglioma. Lack of GFAP immunoreactivity of tumor cells. Note few GFAP-positive cells within the tumor
interpreted as reactive astrocytes (arrows). GFAP-specific polyclonal antibody, ABC method, slightly counterstained with
hematoxylin. Bar ⫽ 30 m. Fig. 6. Spinal cord, canine oligodendroglioma. Factor VIII–related antigen expression of
glomerular-like tufts (arrows). Note lack of immunoreactivity of tumor cells. T ⫽ tumor cells. Factor VII–related antigen
specific antibody, ABC method, slightly counterstained with hematoxylin. Bar ⫽ 30 m.
to moderately reduced posture reactions, neurologic deficits were without significant changes. Myelography showed a
of both forelimbs and hindlimbs, and hyperesthesia between blockage before C5. Because of rapid progression of the
C1 and C7. Computer tomography revealed dilatation of the clinical signs and the poor prognosis, the animal was eu-
lateral ventricles. The disks and bones of the spinal column thanatized and submitted for necropsy.
were without significant changes. The cerebrospinal fluid Gross pathology revealed mild dilatation of the lateral
was clear, and the total leukocyte and red blood cell numbers ventricles and a mild swelling of the cervical spinal cord
Downloaded from vet.sagepub.com at ORILLIA CAMPUS LRC on March 18, 2015
526 Brief Communications and Case Reports Vet Pathol 41:5, 2004
between C3 and C6. No significant macroscopic findings well demarcated, commonly extended into ventricular spaces
were observed in other organs. Tissue samples were fixed in and occur in dogs older than 5 years and are more often
10% buffered formalin, routinely processed, and embedded found in males than in females.4,7 Similarly, in this case a
in paraffin. Four-micron-thick paraffin sections of various subependymal origin of the tumor can be assumed, and the
organs including the spinal cord were cut and stained with tumor also extended into or obliterated the spinal canal.
hematoxylin and eosin and selected slides for luxol fast blue A benign and malignant (anaplastic) oligodendroglioma can
to detect myelin sheaths. For immunohistochemistry anti- be distinguished in domestic animals according to the new
bodies specific for glial fibrillary acidic protein (GFAP: 1 : World Health Organization classification of tumors of the ner-
800), vimentin (1 : 15), cytokeratin (1 : 100), factor VIII–re- vous system.5 In this case, tumor cells were well differentiated,
lated antigen (1 : 200), and the avidin–biotin–peroxidase and the tumor was classified as a benign oligodendroglioma.
complex method with 3,3-diaminobenzidine-tetra-hydro- Dystrophic calcifications, hemorrhages, and necrosis have
chloride-dihydrate were used.1 been reported for most canine oligodendrogliomas.4 Micro-
After formalin fixation a round to oval, 0.3- to 0.8-cm- vascular proliferation is an other characteristic feature of this
diameter, intramedullary mass, which compressed the central tumor. Similarly, prominent neovascularization has been de-
canal was noticed on cross sections of the cervical spinal scribed for high-grade astrocytomas.5,7 Renin and platelet-de-
cord between C5 and C6 (Fig. 1). This well-circumscribed rived growth factor produced by neoplastic astrocytes and
mass was surrounded by compressed spinal cord tissue and vascular endothelium have been implicated as mediators that
exhibited focal areas of hemorrhages. might trigger vasculogenesis.7 Multifocal microcystic areas
Histologically, this cell-rich, well-demarcated, nonencap- and accumulation of mucinous-like material as observed in
sulated tumor occupied about two thirds of the spinal cord this case are also frequent findings in oligodendrogliomas.4,7,8
and compressed the central canal. The tumor consisted of a To summarize, neurologic disturbances and severe ataxia
homogeneous cell population arranged in sheaths or cords correlated well with the anatomic location of this rare spinal
with a fine vascular stroma, areas of multifocal necrosis, cord oligodendroglioma.
hemorrhages, and glomerular-like tufts (Fig. 2). The round
tumor cells were characterized by a moderate amount of a
Acknowledgements
pale basophilic cytoplasm, a round to slightly oval nucleus We would like to thank Mrs. B. Behrens, P. Grünig, and
with fine-stippled heterochromatin, and an inconspicuous K. Rohn for excellent technical and photographic support.
single nucleolus (Fig. 3). No mitotic figures were observed. References
Gitter cells and multifocal dystrophic calcifications were fre-
quently observed within areas of necrosis and hemorrhages. 1 Baumgärtner W, Peixoto PV: Immunohistochemical
Randomly distributed gemistocytes within the tumor and at demonstration of keratin in canine neuroepithelioma. Vet
the periphery of the areas of necrosis were found frequently Pathol 24:500–503, 1987
(Fig. 4). Microcystic areas with accumulation of a pale ba- 2 Forterre F, Kaiser S, Matiasek K, Schmahl W, Brunnberg
sophilic mucinous-like material were seen within the neo- L: Spinal cord canal tumor in dogs: 33 cases (retrospec-
plasm. At the periphery of the tumor, narrow bands of com- tive study). Kleintierpraxis 47:357–364, 2002
pressed gray and white matter were observed. Spinal cord 3 Glimore DR: Intraspinal tumors in the dog. Comp Cont
segments cranial from the neoplasm displayed mild periven- Edu 5:55–64, 1983
tricular malacia, congestion, multifocal hemorrhages, and 4 Koestner A, Higgins RJ: Primary tumors of the central ner-
distortion of the ventral median fissure. No significant his- vous system. In: Tumors in Domestic Animals, ed. Meuten
tologic findings were detected in the remaining spinal cord, DJ, 4th ed., pp. 699–707. Iowa State Press, IA, 2002
brain parenchyma, and other organs. 5 Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers
BA, Van Winkle TJ: Histological classification of tumors
Tumor cells were negative for GFAP, vimentin, cytoker-
of the nervous system of domestic animals. In: WHO
atin, and factor VIII–related antigen. GFAP- and factor VIII–
International Histological Classification of Tumors of
related antigen immunoreactions were found in reactive as-
Domestic Animals, ed. Schulman FY, pp. 13–38. Armed
trocytes, including gemistocytes and endothelial cells of the
Forces Institute of Pathology, Washington, DC, 1999.
glomerular-like tufts, respectively (Figs. 5, 6). The few
6 Prata RG: Diagnosis of spinal cord tumor in the dog. Vet
GFAP-positive cells within the tumor mass with the mor-
Clin North Am 7:165–185, 1977
phology of gemistocytes were interpreted as secondary as-
7 Summers BA, Cummings JF, de Lahunta A. Veterinary
trocytic responses due to tumor-induced tissue damage.
Neuropathology, pp. 363–373. Mosby-Year Book, Inc,
Based on the histologic and immunohistochemical findings
St. Louis, MO, 1995
the neoplasm was diagnosed as an oligodendroglioma.
8 Wilson RB, Beckman SL: Mucinous oligodendroglioma
Common sites of canine oligodendrogliomas in dogs are
of the spinal cord in a dog. J Am Anim Hosp Assoc 31:
the white and gray matter of the cerebral hemispheres and
26–28, 1995
rarely brain stem and spinal cord.4 This neuroectodermal tu-
9 Wright JA: The pathological features associated with spi-
mor has been frequently reported in brachycephalic breeds
nal tumors in 29 dogs. J Comp Pathol 95:549–557, 1985
such as Boxers, Bulldogs, and Boston Terriers.4,7 According
to our literature search, a spinal cord oligodendroglioma has Request reprints from Prof. Wolfgang Baumgärtner, Institut
been reported so far only in a 5-year-old Dachshund dog. In für Pathologie, School of Veterinary Medicine Hannover,
the latter, histology revealed a mucinous variant of this type Bünteweg 17, D-30559 Hannover (Germany). E-mail:
of tumor in the spinal cord.8 Oligodendrogliomas, usually wolfgang.baumgaertner@tiho-hannover.de.
Downloaded from vet.sagepub.com at ORILLIA CAMPUS LRC on March 18, 2015
You might also like
- Spinal Cord Lesions GuideDocument43 pagesSpinal Cord Lesions GuideWorthless Boys100% (4)
- Complex Adnexal TumorDocument2 pagesComplex Adnexal TumorFaduahSalazarNo ratings yet
- Stomeo 2009Document5 pagesStomeo 2009elvirNo ratings yet
- Canine Choroid Plexus Tumor With Intracranial Dissemination Presenting As Multiple Cystic LesionsDocument5 pagesCanine Choroid Plexus Tumor With Intracranial Dissemination Presenting As Multiple Cystic LesionsMihai SavescuNo ratings yet
- Invasive Epithelial Mesothelioma in A Dog: F. R, B. B, K. R, E. S, D. BDocument5 pagesInvasive Epithelial Mesothelioma in A Dog: F. R, B. B, K. R, E. S, D. BjamesyuNo ratings yet
- Mediastinal Mass in A Dog With Syncope and Abdominal DistensionDocument3 pagesMediastinal Mass in A Dog With Syncope and Abdominal DistensionIoana SanduNo ratings yet
- X BibliDocument7 pagesX BibliBJ CarminatorNo ratings yet
- E - Uniform Round Tumour Cells With Small Nucleus and Clear Vacuolated CytoplasmDocument16 pagesE - Uniform Round Tumour Cells With Small Nucleus and Clear Vacuolated CytoplasmAien LeeNo ratings yet
- Laryngeal Plasmacytoma in Kahlers Disease: A Case ReportDocument6 pagesLaryngeal Plasmacytoma in Kahlers Disease: A Case ReportIJAR JOURNALNo ratings yet
- Tall Cell Variant Papillary Thyroid Carcinoma in A Pediatricpatient: A Case Report and Review of The LiteratureDocument6 pagesTall Cell Variant Papillary Thyroid Carcinoma in A Pediatricpatient: A Case Report and Review of The LiteratureIJAR JOURNALNo ratings yet
- Neurocytoma/rhabdomyoma (Myoneurocytoma) of The CerebellumDocument6 pagesNeurocytoma/rhabdomyoma (Myoneurocytoma) of The CerebellumRafael Mujica OreNo ratings yet
- Epithelioid Hemangioendothelioma of The Infundibular-Hypothalamic Region: Case Report and Literature ReviewDocument6 pagesEpithelioid Hemangioendothelioma of The Infundibular-Hypothalamic Region: Case Report and Literature ReviewcarlosNo ratings yet
- Cutaneous ThyroidDocument4 pagesCutaneous ThyroidAlizaPinkyNo ratings yet
- Jurnal IPD 58-11-594 Pericardial MesotheliomaDocument5 pagesJurnal IPD 58-11-594 Pericardial MesotheliomaDulNo ratings yet
- Pilo Matri Coma PosterDocument1 pagePilo Matri Coma Posterkjv5gxdw4fNo ratings yet
- Spindle Cell Lipoma: Franz Enzinger, A. HarveyDocument8 pagesSpindle Cell Lipoma: Franz Enzinger, A. HarveyAdjhy Aji AchmadNo ratings yet
- Desdierencia Entre TFS y Glandula ParotidaDocument7 pagesDesdierencia Entre TFS y Glandula ParotidaReyes Ivan García CuevasNo ratings yet
- Cerebellar Hemangioblastoma-A Rare EntityDocument10 pagesCerebellar Hemangioblastoma-A Rare EntityJay LeheriNo ratings yet
- M: Elaine Crystine Vieira de Paiva Rua Pedro I, 1.033 Centro 60035-101 - Fortaleza - CE - BrazilDocument2 pagesM: Elaine Crystine Vieira de Paiva Rua Pedro I, 1.033 Centro 60035-101 - Fortaleza - CE - Brazilpruebaprueba321765No ratings yet
- 1009 Case 2Document4 pages1009 Case 2willygopeNo ratings yet
- Carcinoma TricoblasticoDocument3 pagesCarcinoma Tricoblasticomilena soriaNo ratings yet
- Cerebellopontine Angle Epidermoid CYST: Case ReportDocument3 pagesCerebellopontine Angle Epidermoid CYST: Case ReportTamajyoti GhoshNo ratings yet
- Intrathyroidal Lymphoepithelial (Branchial) Cyst: Diagnostic and Management Challenge of A Rare EntityDocument5 pagesIntrathyroidal Lymphoepithelial (Branchial) Cyst: Diagnostic and Management Challenge of A Rare EntityMeliNo ratings yet
- CR1.1 - Masriana - Adenocarcinoma of Gallbladder.Document23 pagesCR1.1 - Masriana - Adenocarcinoma of Gallbladder.sarahadila7762No ratings yet
- Cancer de Testiculo e Down 2Document4 pagesCancer de Testiculo e Down 2paulinhamericoNo ratings yet
- A Rare Case of Space Occupying Lesion of Brainstem in An Elderly Male PatientDocument3 pagesA Rare Case of Space Occupying Lesion of Brainstem in An Elderly Male PatientInternational Journal of Clinical and Biomedical Research (IJCBR)No ratings yet
- MCT - Patnaik Grading (1984)Document6 pagesMCT - Patnaik Grading (1984)Yurany RodriguezNo ratings yet
- Follicular Carcinoma of Thyroid Presenting As Brain MetastasisDocument3 pagesFollicular Carcinoma of Thyroid Presenting As Brain MetastasisArif Susilo RahadiNo ratings yet
- Oncovet 2008Document18 pagesOncovet 2008Frederico VelascoNo ratings yet
- Javma-Javma 20 09 0497Document3 pagesJavma-Javma 20 09 0497Fiorella YavarNo ratings yet
- Nigerian Veterinary Journal: Mammary Gland Chondrosarcoma in A German Shepherd Bitch: A Case ReportDocument4 pagesNigerian Veterinary Journal: Mammary Gland Chondrosarcoma in A German Shepherd Bitch: A Case ReportRichard AntiaNo ratings yet
- RPB14150079015Document5 pagesRPB14150079015Ijupbs IjupbsNo ratings yet
- Https:journals Sagepub Com:doi:pdf:10 1354:vp 37-6-650Document3 pagesHttps:journals Sagepub Com:doi:pdf:10 1354:vp 37-6-650Lucas XavierNo ratings yet
- Malignant Myopericytoma: Report of A New Case and Review of The LiteratureDocument6 pagesMalignant Myopericytoma: Report of A New Case and Review of The LiteratureRachel AutranNo ratings yet
- Pleomorphic Sarcoma in Paratesticular Region: Case Report Open AccessDocument5 pagesPleomorphic Sarcoma in Paratesticular Region: Case Report Open AccessNurulDiniaPutriNo ratings yet
- Javma-Javma 20 09 0491Document3 pagesJavma-Javma 20 09 0491Fiorella YavarNo ratings yet
- PROCEEDINGS OF THE PAEDlATRlC NEURO-ONCOLOGY TUMOUR BOARDDocument9 pagesPROCEEDINGS OF THE PAEDlATRlC NEURO-ONCOLOGY TUMOUR BOARDfriiday.qNo ratings yet
- Eye AstrocytomaDocument5 pagesEye AstrocytomacandiddreamsNo ratings yet
- Tumors in InvertebratesDocument19 pagesTumors in InvertebrateshamzaNo ratings yet
- Net Cu Meta in HipofizaDocument6 pagesNet Cu Meta in HipofizaLaura PredescuNo ratings yet
- أنا أتشارك 'pathology' معكDocument3 pagesأنا أتشارك 'pathology' معكMohamed NasserNo ratings yet
- Erbay 2004Document4 pagesErbay 2004Ghani Ikhsan MajidNo ratings yet
- Round Cell TumorsDocument86 pagesRound Cell TumorsMadhura ShekatkarNo ratings yet
- 47 Pathology in PracticeDocument3 pages47 Pathology in PracticeCarlos Alberto Chaves VelasquezNo ratings yet
- Cervical Abnormality of 9 DogsDocument13 pagesCervical Abnormality of 9 DogsRajesh kumarNo ratings yet
- Case Report 2022Document5 pagesCase Report 2022Reyes Ivan García CuevasNo ratings yet
- Tuberculoma of the Brain: Rare Case of Multiple Lesions and Meningeal PlaquesDocument9 pagesTuberculoma of the Brain: Rare Case of Multiple Lesions and Meningeal PlaquessanatthhNo ratings yet
- Atypical Membranoproliferative Glomerulonephritis in A Cat: K. I, J. K - , S. O, S. W, S. M, K. SDocument3 pagesAtypical Membranoproliferative Glomerulonephritis in A Cat: K. I, J. K - , S. O, S. W, S. M, K. SDewa Aix61No ratings yet
- Cervical Sympathetic Chain Ganglioneuroma: Case Report and Review of LiteratureDocument4 pagesCervical Sympathetic Chain Ganglioneuroma: Case Report and Review of LiteratureIOSR Journal of PharmacyNo ratings yet
- Papillary Thyroid Carcinoma: An OverviewDocument6 pagesPapillary Thyroid Carcinoma: An OverviewmarielNo ratings yet
- Neurofibroma, Schwannoma or A Hybrid Tumor of The Peripheral Nerve Sheath?Document4 pagesNeurofibroma, Schwannoma or A Hybrid Tumor of The Peripheral Nerve Sheath?As AsNo ratings yet
- Canine Perineal TumoursDocument11 pagesCanine Perineal TumoursSUSANA SAM RODRIGUEZNo ratings yet
- Hyperoestrogenism and Mammary Adenosis Associated With A Metastatic Sertoli Cell Tumour in A Male Pekingese DogDocument5 pagesHyperoestrogenism and Mammary Adenosis Associated With A Metastatic Sertoli Cell Tumour in A Male Pekingese DogALAN RODRIGO ROJAS COVARRUBIASNo ratings yet
- Shukla The Potential Malignancy of A Solitary Fibrous TumourDocument3 pagesShukla The Potential Malignancy of A Solitary Fibrous TumourJZNo ratings yet
- Somatotroph Pituitary Tumors in Budgerigars (Melopsittacus Undulatus)Document5 pagesSomatotroph Pituitary Tumors in Budgerigars (Melopsittacus Undulatus)Firoz RezaNo ratings yet
- Plasmacytoma of The Thyroid Gland: Case Report and Review of The LiteratureDocument10 pagesPlasmacytoma of The Thyroid Gland: Case Report and Review of The Literaturedede wawaNo ratings yet
- RPM WR2017Document102 pagesRPM WR2017marizamoraNo ratings yet
- Cirugi A Espan Ola: Calcifying Cystic Fibrous Tumour. A Rare Form of Benign Peritoneal CarcinomatosisDocument2 pagesCirugi A Espan Ola: Calcifying Cystic Fibrous Tumour. A Rare Form of Benign Peritoneal CarcinomatosisSani Widya FirnandaNo ratings yet
- HAEMANGIOSARCOMA in DOGSDocument11 pagesHAEMANGIOSARCOMA in DOGSPdea CanineNo ratings yet
- Abdominal Wall Extraskeletal Ewing Sarcoma - Case ReportDocument3 pagesAbdominal Wall Extraskeletal Ewing Sarcoma - Case ReportInternational Organization of Scientific Research (IOSR)No ratings yet
- Case Study of a 23 Year Old Woman with Ewing’s SarcomaDocument2 pagesCase Study of a 23 Year Old Woman with Ewing’s SarcomaSoleil SierraNo ratings yet
- Rare Association of A c1-c2 Schwannoma in Pregnant Women About A Case and Literature ReviewDocument4 pagesRare Association of A c1-c2 Schwannoma in Pregnant Women About A Case and Literature ReviewCelebre MualabaNo ratings yet
- Spinal Tumors - A Brief OverviewDocument38 pagesSpinal Tumors - A Brief OverviewRAVIRAJ GHORPADE BELGAUM ADVANCED NEUROSURGERYNo ratings yet
- Dr. YSR Aarogyasri Health Care Trust 4Document16 pagesDr. YSR Aarogyasri Health Care Trust 4saiprashanth5.11No ratings yet
- Report of Three Cases With Rare Spinal Epidural TumorsDocument1 pageReport of Three Cases With Rare Spinal Epidural TumorsHaris GiannadakisNo ratings yet
- (Journal of Neurosurgery - Spine) The Lateral Extracavitary Approach To The Thoracolumbar Spine - A Case Series and Systematic ReviewDocument10 pages(Journal of Neurosurgery - Spine) The Lateral Extracavitary Approach To The Thoracolumbar Spine - A Case Series and Systematic Reviewkarine cim assençoNo ratings yet
- Intradural Spinal Tumors in Adults - Update On Management and OutcomeDocument18 pagesIntradural Spinal Tumors in Adults - Update On Management and OutcomeSuciatije PenelitianNo ratings yet
- Spinal Tumor StrongDocument45 pagesSpinal Tumor StrongbettyNo ratings yet
- Pi Is 0025619611619654Document10 pagesPi Is 0025619611619654Ivo Baaron ZarkovNo ratings yet
- Atlas of PET CT in Oncology - Volume 1 Brain Head and Neck Cancers Yao 1 Ed 2023Document322 pagesAtlas of PET CT in Oncology - Volume 1 Brain Head and Neck Cancers Yao 1 Ed 2023martinus.urbanusNo ratings yet
- Spinal Cord TumorsDocument7 pagesSpinal Cord TumorsVALERIA TEJEDA SANCHEZNo ratings yet
- Carotid CavernoidDocument13 pagesCarotid Cavernoiduyeee aulNo ratings yet
- Cong Anomalies and Tumors of SpineDocument120 pagesCong Anomalies and Tumors of SpineSunny SbaNo ratings yet
- Tan Ah Kau V The Government of Malaysia - (2Document17 pagesTan Ah Kau V The Government of Malaysia - (2Arvin ANo ratings yet
- In Braunds Clinical Neurology in Small Animals LoDocument24 pagesIn Braunds Clinical Neurology in Small Animals LoNxyz GxyzNo ratings yet
- Intradural Spinal Cord Tumors: UpplementDocument1 pageIntradural Spinal Cord Tumors: Upplementlauzark gashbellNo ratings yet
- Chan 2016Document8 pagesChan 2016Samuel MonsalveNo ratings yet
- PosterDocument1 pagePosterRaif RizqullahNo ratings yet
- Spinal Surgery Laminectomy Fusion AHM 1Document14 pagesSpinal Surgery Laminectomy Fusion AHM 1Rashmi ShriNo ratings yet
- Spinal TumorsDocument59 pagesSpinal TumorsAl-Banji MohammadNo ratings yet
- Spinal Cord Disease by GadisaDocument95 pagesSpinal Cord Disease by GadisaGadisa DejeneNo ratings yet
- 1354 4486 2 PBDocument198 pages1354 4486 2 PBlinda.e.larisaNo ratings yet
- Tumors of SpineDocument13 pagesTumors of Spinebosnia agusNo ratings yet
- Intradural Extramedullary Spinal Tumors GuideDocument4 pagesIntradural Extramedullary Spinal Tumors Guideabishek22No ratings yet
- Spinal Cord TumoursDocument15 pagesSpinal Cord TumoursSakshi NegiNo ratings yet
- Ebook MriDocument26 pagesEbook MrikadekNo ratings yet
- Pre-Revised and Revised Procedure Charges From Jan 1, 2016Document26 pagesPre-Revised and Revised Procedure Charges From Jan 1, 2016Ashish Singh NegiNo ratings yet
- Not All Twitching Is Epileptic! Hand Myoclonus in A Boy WithDocument2 pagesNot All Twitching Is Epileptic! Hand Myoclonus in A Boy WithVincentius Michael WilliantoNo ratings yet
- Neuroscience Ii: Summary: Nationality (Will Tell You Incidence, For Example, AsiansDocument29 pagesNeuroscience Ii: Summary: Nationality (Will Tell You Incidence, For Example, AsiansAngelaTrinidad100% (2)