Professional Documents
Culture Documents
Session 7 Topic Peritectic Phase Diagram
Session 7 Topic Peritectic Phase Diagram
Uploaded by
Thaya Ganapathy0 ratings0% found this document useful (0 votes)
9 views3 pagesOriginal Title
Session 7 topic Peritectic Phase Diagram
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views3 pagesSession 7 Topic Peritectic Phase Diagram
Session 7 Topic Peritectic Phase Diagram
Uploaded by
Thaya GanapathyCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 3
O Like the eutectic system, the peritectic reaction is found in systems with complete liquid
solubility but limited solid solubility.
© Inthe peritectic reaction the liquid (L) reacts with one solid («) to produce another solid (B)..
rL+a3B.
GF Since the solid B forms at the interface between the L and the a, finther reaction is
dependent on solid state diffusion. Needless to say this becomes the rate limiting step and
hence it is difficult to ‘equilibrate’ Peritectic reactions (as compared to say eutectic
reactions). Aoriim.
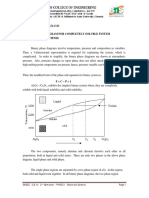
‘In some peritectic reactions (¢.g. the Pt-Ag system next page), the (pure) B pase is not
stable below the peritectic temperature (T, = 1186°C for Pt-Ag system) and splits into a
mixture of (a + B) just below Tp.
that bow Ty
faand spits mie (a=
os fad 66.3,
Pe TP 20 30. 4S 50 oo Xo
80 90 Ag
a
663%de "10.5% AE wwe 424% AG
cr
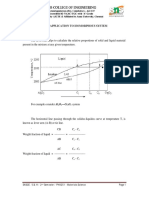
Eutectoid & Peritectic — some definitions
= Eutectic: a liquid in equilibrium with two solids
L sharp
= Eutectoid: solid phase in equilibrium with two solid phases
S, == S,t+S; intermetalic compound cementite
7 2 atFet (727°C)
reat
= Peritecti id + solid ibrium with a single solid
2 (Fig 9.21)
Sith = & cool
StL oY (1493°C)
You might also like
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Unit I MCQDocument18 pagesUnit I MCQThaya GanapathyNo ratings yet
- Binary Phase Diagrams: Binary Phase Diagram For Completely Soluble System (Isomorphous Systems)Document2 pagesBinary Phase Diagrams: Binary Phase Diagram For Completely Soluble System (Isomorphous Systems)Thaya GanapathyNo ratings yet
- Session 5 Topic 5 Isomorphous Systems, The Tie-Line RuleDocument2 pagesSession 5 Topic 5 Isomorphous Systems, The Tie-Line RuleThaya GanapathyNo ratings yet
- Modern Engineering Materials: Unit - VDocument11 pagesModern Engineering Materials: Unit - VThaya GanapathyNo ratings yet
- Microstructure of A Lead-Tin AlloyDocument55 pagesMicrostructure of A Lead-Tin AlloyThaya GanapathyNo ratings yet
- Physics For Bioscience: Rajalakshmi Engineering College (Autonomous) Thandalam, Chennai - 602105Document193 pagesPhysics For Bioscience: Rajalakshmi Engineering College (Autonomous) Thandalam, Chennai - 602105Thaya GanapathyNo ratings yet
- PH8251-Material Science QN Bank ValliammaiDocument10 pagesPH8251-Material Science QN Bank ValliammaiThaya GanapathyNo ratings yet
- Topic 2.1 Oscillatory Motion: Department of Science and Humanities MCQ For Regulations 2017Document13 pagesTopic 2.1 Oscillatory Motion: Department of Science and Humanities MCQ For Regulations 2017Thaya GanapathyNo ratings yet
- Unit Iii Thermal Physics: Department of Science and Humanities MCQ For Regulations 2017Document19 pagesUnit Iii Thermal Physics: Department of Science and Humanities MCQ For Regulations 2017Thaya GanapathyNo ratings yet
- Question Bank US03CPHY01 Unit1 To 4 Optics PMPDocument13 pagesQuestion Bank US03CPHY01 Unit1 To 4 Optics PMPThaya GanapathyNo ratings yet
- 10 - Chapter 1Document24 pages10 - Chapter 1Thaya GanapathyNo ratings yet
- Solid State Physics 1 PGDocument4 pagesSolid State Physics 1 PGThaya GanapathyNo ratings yet
- 08 - Chapter 1Document19 pages08 - Chapter 1Thaya GanapathyNo ratings yet