Professional Documents
Culture Documents
Session 5 Topic 5 Isomorphous Systems, The Tie-Line Rule
Session 5 Topic 5 Isomorphous Systems, The Tie-Line Rule
Uploaded by
Thaya GanapathyOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Session 5 Topic 5 Isomorphous Systems, The Tie-Line Rule
Session 5 Topic 5 Isomorphous Systems, The Tie-Line Rule
Uploaded by
Thaya GanapathyCopyright:
Available Formats
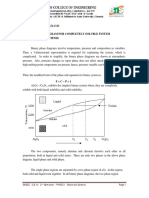
THE LEVER RULES, APPLICATION TO ISOMORPHOUS SYSTEM
The Lever rule
The lever rule helps to calculate the relative proportions of solid and liquid material
present in the mixture at any given temperature.
For example consider Al2O3—Cr2O3 system
The horizontal line passing through the solidus-liquidus curve at temperature T, is
known as lever arm (A-B) or tie line.
` CB Cs - Co
Weight fraction of liquid = ----------- = ------------------
AB Cs - Ci
AC Co - Cl
Weight fraction of liquid = ----------- = ------------------
AB Cs - Cl
SNSCE /S & H / 2nd Semester / PH8251 - Materials Science Page 1
SNS COLLEGE OF ENGINEERING, Coimbatore
Then, the lever rule states that, to determine the overall composition of solid and liquid
fractions at point C, (Temperature T), consider that point as fulcrum and ends as weights
(weight fraction of solid and liquid). At temperature T, at particular composition of chromia
at Co (point C), the fraction of liquid (point A, Cl) and weight fraction of solid (point B, Cs)
is given by the above equation.
The lever rule is not applicable at eutectic or peritectic points as three phases exist in
equilibrium at these temperatures. It can be applied just below or just above the invariant
line. The lever rule may be used to calculate
i) The fraction of a proeutectic phase
ii) The fraction of an eutectic mixture, and
iii) The fraction of phase of an eutectic mixture.
Applications of isomorphous systems (phase diagrams)
Alloy phase diagrams are useful to metallurgists, materials engineers, and materials scientists
in the following major areas:
Development of new alloys for specific applications,
Fabrication of these alloys into useful configurations,
Design and control of heat treatment procedures for specific alloys that will
produce the required mechanical, physical, and chemical properties, and
Solving problems that arise with specific alloys in their performance in
commercial applications, thus improving product predictability
Predict the temperature at which freezing or melting begins or ends for any specific
alloy composition in an alloy system.
Predict the safe temperature for hot working or heat treatment
Determine the number of phases, type of phases, and composition of phases present in
any given alloy at a specific temperature.
Predict possible heat treatments.
Alloy Design in age-hardening alloys, forming pure austenite steel phase, to get
permanent magnetic phase
SNSCE /S & H / 2nd Semester / PH8251 - Materials Science Page 2
You might also like
- Credit Card DetailsDocument4 pagesCredit Card Detailsabdou allalou0% (2)
- Afbeeldingen: Full Galar Map With DLCDocument1 pageAfbeeldingen: Full Galar Map With DLCGarron PardoNo ratings yet
- Lead Tin Phase Diagram ExperimentDocument5 pagesLead Tin Phase Diagram ExperimentOliver Tabell100% (2)
- Is Starting A Business Right For YouDocument33 pagesIs Starting A Business Right For YouSorom ComputerNo ratings yet
- Question & Answer Set-7Document12 pagesQuestion & Answer Set-7eeng.ali651550% (2)
- Consumer Relations Case-9Document29 pagesConsumer Relations Case-9Ameera MazlanNo ratings yet
- Module - 3, Iron - Carbon SystemDocument43 pagesModule - 3, Iron - Carbon SystemSayaf Khan PathanNo ratings yet
- Chapter 4-Phase DiagramDocument16 pagesChapter 4-Phase Diagramtky96No ratings yet
- Phase DiagramsDocument21 pagesPhase DiagramsJnrNo ratings yet
- CHAPTER 3 Phase Diagram TTT HT - 1stDocument25 pagesCHAPTER 3 Phase Diagram TTT HT - 1stAriff AziziNo ratings yet
- New Civil Module5 PDF NotesDocument18 pagesNew Civil Module5 PDF NotesDrMohan KumarNo ratings yet
- Phase Diagram ExperimentDocument8 pagesPhase Diagram ExperimentAnand PatelNo ratings yet
- Chapter8 PhaseDiagram HandoutsDocument27 pagesChapter8 PhaseDiagram Handoutswagdy87No ratings yet
- Chapter 4Document68 pagesChapter 4Ermias GuragawNo ratings yet
- Aluminum Extrusion FsiDocument9 pagesAluminum Extrusion FsiXPTO_COMNo ratings yet
- Module 3, Iron - Carbon SystemDocument48 pagesModule 3, Iron - Carbon Systemtanishka narayanNo ratings yet
- Phase Diagrams: The Beginning of WisdomDocument26 pagesPhase Diagrams: The Beginning of WisdomOlga Sandoval RomeroNo ratings yet
- Diagramas de FaseDocument44 pagesDiagramas de Faserandomspamacc4No ratings yet
- 3D Conjugate Heat Transfer Analysis of A 100 KN Class Liquid Rocket Combustion ChamberDocument13 pages3D Conjugate Heat Transfer Analysis of A 100 KN Class Liquid Rocket Combustion ChamberAli mohammad bagheriNo ratings yet
- Lecture n.15 Phase DiagramsDocument24 pagesLecture n.15 Phase DiagramsMOHAMMAD JAWAD QASIMNo ratings yet
- Engineering Materials 24-26Document31 pagesEngineering Materials 24-26Sanu SouravNo ratings yet
- Phase Diagrams For Metallic SystemsDocument5 pagesPhase Diagrams For Metallic SystemsZesi Villamor Delos SantosNo ratings yet
- Part 9 - Phase DiagramsDocument10 pagesPart 9 - Phase DiagramsIsaac YoonNo ratings yet
- Final Lect5 PhaseDocument24 pagesFinal Lect5 Phasesayedshoaib2004No ratings yet
- J. Chem. Thermodynamics: C. Costa, S. Delsante, G. Borzone, D. Zivkovic, R. NovakovicDocument12 pagesJ. Chem. Thermodynamics: C. Costa, S. Delsante, G. Borzone, D. Zivkovic, R. NovakovicCiubotaru Andrei-GabrielNo ratings yet
- List of Topics Ordering Online Manual Download Demo Updates Product Endorsements Award Review Materials Science On CD-ROM User GuideDocument8 pagesList of Topics Ordering Online Manual Download Demo Updates Product Endorsements Award Review Materials Science On CD-ROM User GuideEveraldo FernandesNo ratings yet
- Formal Lab 2Document38 pagesFormal Lab 2sreyes4100% (1)
- Lab 7 - Phase DiagramsDocument7 pagesLab 7 - Phase Diagramsabd333No ratings yet
- Estimation of Corrosion Kinetics ParametersDocument16 pagesEstimation of Corrosion Kinetics ParametersFelipe Cepeda SilvaNo ratings yet
- SPE 132455 A Practical Approach For Calculating EOS CoefficientsDocument25 pagesSPE 132455 A Practical Approach For Calculating EOS CoefficientsMohamed ElkumatiNo ratings yet
- Emm HandoutsDocument58 pagesEmm HandoutsDr.A.Maniram KumarNo ratings yet
- 1.1 Diagramas de FasesDocument60 pages1.1 Diagramas de FasesAlex Pelinco ApazaNo ratings yet
- Chapter 8 Phase Diagrams UpdatedDocument80 pagesChapter 8 Phase Diagrams UpdatedSalman Khalil100% (1)
- Batch CSTR ExperimentDocument5 pagesBatch CSTR ExperimentNaeem YounisNo ratings yet
- 16 970396970Document15 pages16 970396970valeska reinosoNo ratings yet
- Science of Materials Science of Materials C: Dr. Andres MarquezDocument31 pagesScience of Materials Science of Materials C: Dr. Andres MarquezAPNo ratings yet
- Unit-2: Phase DiagramDocument37 pagesUnit-2: Phase DiagramPrasad Govind KumbharNo ratings yet
- Week 9 10 - 1 - IPE 2203-LecturesDocument86 pagesWeek 9 10 - 1 - IPE 2203-LecturesMD Al-AminNo ratings yet
- Rules in MetallurgyDocument16 pagesRules in Metallurgytejaslilhore59No ratings yet
- Chapter 9 ReportDocument16 pagesChapter 9 ReportG. Dancer GhNo ratings yet
- Mathematical Modelling of Steel QuenchingDocument6 pagesMathematical Modelling of Steel Quenchingmanashree02No ratings yet
- TTOPIC 6 Phase PDFDocument19 pagesTTOPIC 6 Phase PDFSaud AssiriNo ratings yet
- Some Aspects of Theory and Mathematics O F Thermal Runaway in Reacting Chemical Systems That May Be Relevant To Critical Behaviour in ConfinedDocument3 pagesSome Aspects of Theory and Mathematics O F Thermal Runaway in Reacting Chemical Systems That May Be Relevant To Critical Behaviour in ConfinedKamel IbrahimNo ratings yet
- CFD Study of Orifice Pulse Tube Cryo-Cooler: B. S. Gawali, P. A. ManeDocument6 pagesCFD Study of Orifice Pulse Tube Cryo-Cooler: B. S. Gawali, P. A. ManeksvvijNo ratings yet
- Phase Diagrams and Phase TransformationsDocument38 pagesPhase Diagrams and Phase TransformationsNameIs RajNo ratings yet
- Phase Equilibria and Phase TransformationDocument57 pagesPhase Equilibria and Phase TransformationAzhan Haqimi100% (1)
- 4Document63 pages4ARJUN PESARU 19BME0161No ratings yet
- The Thermal Behaviour 0F Overhead ConductorsDocument14 pagesThe Thermal Behaviour 0F Overhead ConductorsjuanperezpintoNo ratings yet
- Phase DiagramsDocument50 pagesPhase DiagramsIbrahim MalikNo ratings yet
- Kalnins 2005Document8 pagesKalnins 2005bahmanNo ratings yet
- Tutorial 3 SolutionDocument5 pagesTutorial 3 SolutionUmmi Kalthum MohamadNo ratings yet
- Phase Diagrams & Heat Treatment of Carbon SteelDocument84 pagesPhase Diagrams & Heat Treatment of Carbon SteelTanmay DuttaNo ratings yet
- 114 AnnesiniDocument6 pages114 AnnesiniRizky KusumastutiNo ratings yet
- Fraser-Hacker2008 Article AnEmpiricalFuelCellPolarizatioDocument6 pagesFraser-Hacker2008 Article AnEmpiricalFuelCellPolarizatioSeb NeoNo ratings yet
- Metallurgical EngineeringDocument49 pagesMetallurgical EngineeringMatab ThamerNo ratings yet
- Phase Diagram 1Document23 pagesPhase Diagram 1Tony StarkNo ratings yet
- Ternary Phase DiagramDocument9 pagesTernary Phase DiagramFabiha SheikhNo ratings yet
- Chapter 9Document57 pagesChapter 9Pavan PonnadaNo ratings yet
- Ie 00023 A 033Document9 pagesIe 00023 A 033Daiane FreitasNo ratings yet
- Re CrystallizationDocument5 pagesRe CrystallizationZubair AhmadNo ratings yet
- Material Engineering QUESTION BANKDocument13 pagesMaterial Engineering QUESTION BANKFUNTUBENo ratings yet
- Unit 4Document67 pagesUnit 4pthangarasu sctengNo ratings yet
- Chapter On Nozzle TheoryDocument57 pagesChapter On Nozzle TheoryPrabhjot Singh Sahi50% (2)
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Question Bank US03CPHY01 Unit1 To 4 Optics PMPDocument13 pagesQuestion Bank US03CPHY01 Unit1 To 4 Optics PMPThaya GanapathyNo ratings yet
- Binary Phase Diagrams: Binary Phase Diagram For Completely Soluble System (Isomorphous Systems)Document2 pagesBinary Phase Diagrams: Binary Phase Diagram For Completely Soluble System (Isomorphous Systems)Thaya GanapathyNo ratings yet
- Microstructure of A Lead-Tin AlloyDocument55 pagesMicrostructure of A Lead-Tin AlloyThaya GanapathyNo ratings yet
- PH8251-Material Science QN Bank ValliammaiDocument10 pagesPH8251-Material Science QN Bank ValliammaiThaya GanapathyNo ratings yet
- Modern Engineering Materials: Unit - VDocument11 pagesModern Engineering Materials: Unit - VThaya GanapathyNo ratings yet
- Topic 2.1 Oscillatory Motion: Department of Science and Humanities MCQ For Regulations 2017Document13 pagesTopic 2.1 Oscillatory Motion: Department of Science and Humanities MCQ For Regulations 2017Thaya GanapathyNo ratings yet
- Physics For Bioscience: Rajalakshmi Engineering College (Autonomous) Thandalam, Chennai - 602105Document193 pagesPhysics For Bioscience: Rajalakshmi Engineering College (Autonomous) Thandalam, Chennai - 602105Thaya GanapathyNo ratings yet
- Unit I MCQDocument18 pagesUnit I MCQThaya GanapathyNo ratings yet
- Unit Iii Thermal Physics: Department of Science and Humanities MCQ For Regulations 2017Document19 pagesUnit Iii Thermal Physics: Department of Science and Humanities MCQ For Regulations 2017Thaya GanapathyNo ratings yet
- Solid State Physics 1 PGDocument4 pagesSolid State Physics 1 PGThaya GanapathyNo ratings yet
- Electricity and MagnetismDocument275 pagesElectricity and MagnetismThaya Ganapathy100% (3)
- 10 - Chapter 1Document24 pages10 - Chapter 1Thaya GanapathyNo ratings yet
- 08 - Chapter 1Document19 pages08 - Chapter 1Thaya GanapathyNo ratings yet
- Exam1 SolutionsDocument4 pagesExam1 SolutionsThaya GanapathyNo ratings yet
- 11 Fluids 1Document13 pages11 Fluids 1Thaya GanapathyNo ratings yet
- Atg UvlaDocument1 pageAtg UvlanattaponamornNo ratings yet
- Give Me A Scout: (Contributed by Allan Schup, Scoutmaster, Troop 502, Longhorn CouncilDocument5 pagesGive Me A Scout: (Contributed by Allan Schup, Scoutmaster, Troop 502, Longhorn CouncilbrfundNo ratings yet
- Engineering Drawing and Graphics Basant Agrawal C M Agrawal Multiple Choice QuestionsDocument3 pagesEngineering Drawing and Graphics Basant Agrawal C M Agrawal Multiple Choice QuestionsMrityunjay KrNo ratings yet
- Practical 5Document6 pagesPractical 5sahil singhaniaNo ratings yet
- GCASH ReportingDocument16 pagesGCASH ReportingSam ReigoNo ratings yet
- Performance Qualification Protocol Vial Washing MachineDocument17 pagesPerformance Qualification Protocol Vial Washing MachineBirol ErgenNo ratings yet
- Creating A Diagram of The Film Company LANDocument3 pagesCreating A Diagram of The Film Company LANonlycisco.tkNo ratings yet
- Brand Appeal and Customers' Patronage of Fast Food Firms in Port HarcourtDocument14 pagesBrand Appeal and Customers' Patronage of Fast Food Firms in Port HarcourtMhiz AdesewaNo ratings yet
- Faculty and Staff Meeting-ChineseDocument3 pagesFaculty and Staff Meeting-ChineseMargaux Faith CCNo ratings yet
- Flipped Classroom Lesson Plan TemplateDocument2 pagesFlipped Classroom Lesson Plan Templateapi-351598518No ratings yet
- Slackline Training For Balance and Strength PromotionDocument7 pagesSlackline Training For Balance and Strength PromotionGuilherme CaetanoNo ratings yet
- Youth For AgricultureDocument15 pagesYouth For Agricultureaeli.resareNo ratings yet
- Settlement CalculationDocument16 pagesSettlement Calculationalicarlos13No ratings yet
- 14000Document5 pages14000umarulafzanNo ratings yet
- MAKS Self Assessment ToolDocument4 pagesMAKS Self Assessment ToolShahryar SaqibNo ratings yet
- Lesson 15 - 2Document39 pagesLesson 15 - 2Sopphia CalopeNo ratings yet
- Design and Fabrication of Amphibious Bicycle: June 2015Document36 pagesDesign and Fabrication of Amphibious Bicycle: June 2015Amanuel GetachewNo ratings yet
- TransformersDocument2 pagesTransformersNithya GowdaNo ratings yet
- Tiar Pramono PDFDocument9 pagesTiar Pramono PDFTiar PramonoNo ratings yet
- MDE8051 ToggleDocument1 pageMDE8051 TogglepratikNo ratings yet
- G2A Autopilot MONEY MAKING METHOD + BonusDocument68 pagesG2A Autopilot MONEY MAKING METHOD + Bonusmake easy money0% (1)
- Untermann, 1999Document7 pagesUntermann, 1999RAFIQNo ratings yet
- Interview: Place of Interview FPSC Provincial Office, 31-Civic Centre, Mustafa Town, Wahdat Road, LahoreDocument4 pagesInterview: Place of Interview FPSC Provincial Office, 31-Civic Centre, Mustafa Town, Wahdat Road, LahoreShan ZaibNo ratings yet
- What Do You WhatDocument22 pagesWhat Do You WhatSaikat SenguptaNo ratings yet
- Anthony PDFDocument33 pagesAnthony PDFQuang ThangNo ratings yet
- Minor Project Report-OSPF ChampDocument5 pagesMinor Project Report-OSPF Champsmrutiranjan1991100% (1)