Professional Documents
Culture Documents
Metabolism Part 4 With Audio
Uploaded by
Acel Jone Cayot0 ratings0% found this document useful (0 votes)
6 views18 pagesCopyright
© © All Rights Reserved
Available Formats
TXT, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as TXT, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views18 pagesMetabolism Part 4 With Audio
Uploaded by
Acel Jone CayotCopyright:
© All Rights Reserved
Available Formats
Download as TXT, PDF, TXT or read online from Scribd
You are on page 1of 18
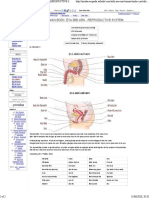
ELECTRON TRANSPORT CHAIN
– A series of biochemical reactions in which
electrons and hydrogen ions from NADH and
FADH2 are passed to intermediate carriers
and then ultimately react with molecular
oxygen to form water.
– NADH and FADH2 are oxidized in the process.
ELECTRON TRANSPORT CHAIN
– Electrons passed in each step of ETC lose some
energy.
– Some of these lost energy are used to make ATP
from ADP.
– Inner mitochondial membrane- enzymes and
electron carriers are located.
PROTEIN COMPLEXES:
Bound to membrane
❑COMPLEX I: NADH-coenzyme Q reductase
❑COMPLEX II: Succinate-coenzyme Q reductase
❑COMPLEX III: Coenzyme Q-cytochrome c reductase
❑COMPLEX IV: Cytochrome c oxidase
❑Coenzyme Q and Cytochrome -mobile electron carriers
COMPLEX I
– COMPLEX I: NADH-coenzyme Q reductase (NADH→ CoQ)
– LARGEST (40 subunits)
– FMN and Fe-S proteins (FESP)
STEPS:
1. Interaction of NADH with FMN.
– NAD is oxidized to NAD+, passes 2 H ions and e to FMN, forming
FMNH2
2. Transfer of e from FMNH2 through Fe-S proteins.
– FMNH2 releases 2 H ions to the solution.
– This needs 2 Fe-SP molecules to accommodate the release.
COMPLEX I
STEPS:
3. Fe(II)SP is reconverted into Fe(III)SP, as each two Fe(II)SP
units passes an e to CoQ (CoQ to CoQH2).
– CoQ and CoQH2 are lipid soluble and can move laterally
within the mitochondrial membrane.
– CoQH2 shuttles e to COMPLEX III.
COMPLEX III
– 11 subunits
– Contains FeSPs and Cytochromes
– Cytochrome: heme-containing protein in which
reversible oxidation and reduction of an iron atom
occur.
COMPLEX III
STEPS:
1. Oxidation of CoQH2 to CoQ
– 2H+ produced go into cellular solution.
– Electron transport proceeds from CoQH2 to an FeSP, to cyt
b, then to another FeSP, to cyt c1, and finally to cyt c.
– Cyt c can move laterally in the intermembrane space,
delivers its e to COMPLEX IV.
– Cyt c is the only water-soluble cytochrome.
COMPLEX IV
– 13 subunits, including 2 cytochromes
– Cyt c to Cyt a, then cyt a3.
– Electrons from cyt a3 and H+ ions from cellular solution
combine with Oxygen to form water.
– 95% of the oxygen used by cells serve as the final electron
acceptor for the ETC.
COMPLEX II
– 4 subunits, including 2 FESP molecules.
– Process FADH2
– CoQ is associated with the operations in COMPLEX II in a
manner similar to its actions in COMPLEX I.
– CoQ is the final recipient of the e from FADH2 (FeSP as
intermediaries).
– Complex I and II both produce CoQH2.
OXIDATIVE PHOPHORYLATION
– A biochemical process by which ATP is synthesized from ADP as
a result of the transfer of electrons and hydrogen ions from
NADH or FADH2 to O2 through the electron carriers involved in
the ETC.
– Couple reactions: pairs of biochemical reactions that occur
concurrently in which energy released by one reaction is used in
the other reaction.
– Oxidative phosphorylation and oxidation reactions of ETC are
coupled reactions.
OXIDATIVE PHOPHORYLATION
– COMPLEX I, III, & IV are also known as proton pumps
– For every 2 e- passed through ETC, 4 protons cross the
inner membrane through Complex I, 4 protons through
Complex III, and 2 through Complex IV.
– Proton flows causes buildup of H+ ions in the
intermembrane space.
– This high concentration of protons becomes the basis for
ATP synthesis.
OXIDATIVE PHOPHORYLATION
– Relationship of electron carriers and number of protons:
– Oxidation of NADH= 10 protons crossing the membrane
– Oxidation of FADH2= 6 protons crossing the membrane
– Proton flow= explained through CHEMIOSMOTIC COUPLING.
– CHEMIOSMOTIC COUPLING: coupling of ATP synthesis with ETC
reactions require a proton gradient across the inner mitochondrial
membrane.
OXIDATIVE PHOPHORYLATION
– Electrochemical gradient- electrical charge ion (proton H+) is higher
in concentration in the intermembrane space than in matrix.
– Spontaneous flow of protons from high concentration to low
concentration is facilated by ATPS synthase (inner mitochondrial
membrane).
– The flow powers up the synthesis of ATP, thus ATPS synthases are
coupling factors that link the OP and ETC.
OXIDATIVE PHOPHORYLATION
ATP Synthase
– 2 subunits: F0 and F1
– F0= channel of the proton flow
– F1=where formation of ATP takes place.
– The potential energy associated with the electrochemical
gradient is released and used in the F1 subunit for the synthesis
of ATP.
OXIDATIVE PHOPHORYLATION
– ATP must be moved from the matrix to the intermembrane
space before it can be used in the metabolic reactions.
– ATP is transported through the transport proteins
imbedded in the inner mitochondrial membrane.
OXIDATIVE PHOPHORYLATION
– 1 ATP molecule transported out of the matrix=
1 ADP
1 Pi
1 H+
Move in the opposite direction.
TAKE NOTE: 4 H+ powers the ATP synthesis
3 H+ from the gradient, 1 H+ as a result of movement of ATP out of the
matrix.
ATP PRODUCTION
– 1 NADH= pumps 10 protons= 2.5 moles ATP
– 3 NADH (1 cycle of TCA)=30 protons= 7.5 moles ATP
– 1 FADH2= pumps 6 protons= 1.5 moles ATP
– 1 GTP= 1 mole ATP
– TOTAL= 10 ATP
You might also like
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (589)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5806)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Harpers Illustrated Biochemistry 32nd Ed. Exams Answer KeyDocument50 pagesHarpers Illustrated Biochemistry 32nd Ed. Exams Answer KeyTeddy MahusayNo ratings yet
- Biology NotesDocument225 pagesBiology NotesShaurya GuptaNo ratings yet
- Probiotic Research in Therapeutics: Indu Pal Kaur Editor-in-Chief Parneet Kaur Deol EditorDocument373 pagesProbiotic Research in Therapeutics: Indu Pal Kaur Editor-in-Chief Parneet Kaur Deol EditorGepetto ArtsNo ratings yet
- Week 1 - HEMATOPOIESISDocument8 pagesWeek 1 - HEMATOPOIESISAcel Jone CayotNo ratings yet
- Protective - Coating Lipids Biological WaxesDocument11 pagesProtective - Coating Lipids Biological WaxesAcel Jone CayotNo ratings yet
- Week 3 - HemoglobinDocument4 pagesWeek 3 - HemoglobinAcel Jone CayotNo ratings yet
- Z Messenger Lipids Steroid HormonesDocument10 pagesZ Messenger Lipids Steroid HormonesAcel Jone CayotNo ratings yet
- Urea Cycle Prepared By: CST, RMT 2020Document30 pagesUrea Cycle Prepared By: CST, RMT 2020Acel Jone CayotNo ratings yet
- Protein Metabolism Part 1 Prepared By: CST, RMT 2020Document44 pagesProtein Metabolism Part 1 Prepared By: CST, RMT 2020Acel Jone CayotNo ratings yet
- Diphenylamin e Test For Deoxyribose By: Anne Marielle D. ManaloDocument17 pagesDiphenylamin e Test For Deoxyribose By: Anne Marielle D. ManaloAcel Jone CayotNo ratings yet
- 1Document2 pages1Acel Jone CayotNo ratings yet
- Biochemistry of Nucleic AcidsDocument12 pagesBiochemistry of Nucleic AcidsAcel Jone CayotNo ratings yet
- Only The Capacitated Spermatozoa Undergo Acrosomal ReactionDocument7 pagesOnly The Capacitated Spermatozoa Undergo Acrosomal ReactionbarbacumlaudeNo ratings yet
- Microbiological Examination of Nonsterile Cannabis Products - 8 - 17 - 2018Document60 pagesMicrobiological Examination of Nonsterile Cannabis Products - 8 - 17 - 2018Reza FebryantaraNo ratings yet
- Reproduction in Lower and Higher PlantsDocument89 pagesReproduction in Lower and Higher Plantsmohdyasir6643No ratings yet
- World Psychiatry - 2023 - Abi Dargham - Candidate Biomarkers in Psychiatric Disorders State of The FieldDocument27 pagesWorld Psychiatry - 2023 - Abi Dargham - Candidate Biomarkers in Psychiatric Disorders State of The FieldPranjal Sharma RNo ratings yet
- Heredity Revision WorksheetDocument4 pagesHeredity Revision WorksheetkaylatheunicornytNo ratings yet
- Nure Seminar 0910114238Document38 pagesNure Seminar 0910114238ABAYNEGETAHUN getahunNo ratings yet
- Cell Biology Test - 70 Possible Points: Prokaryotic and Eukaryotic Cells (2 Points Per Question)Document3 pagesCell Biology Test - 70 Possible Points: Prokaryotic and Eukaryotic Cells (2 Points Per Question)Vienne MonroidNo ratings yet
- Module 6 RationaleDocument1 pageModule 6 RationaleG INo ratings yet
- Introduction To The Immune SystemDocument21 pagesIntroduction To The Immune SystemDiandra DwitavianyNo ratings yet
- Ginting Et Al 2019 Efficacy and Residual Activity of Lecanicillium Kalimantanense FINAL PDFDocument9 pagesGinting Et Al 2019 Efficacy and Residual Activity of Lecanicillium Kalimantanense FINAL PDFRestiNo ratings yet
- Bifidobacterium Asteroides PRL2011 Genome Analysis Reveals Clues For Colonization of The Insect GutDocument14 pagesBifidobacterium Asteroides PRL2011 Genome Analysis Reveals Clues For Colonization of The Insect Guttissue88No ratings yet
- Paperclip Activity-Dna ReplicationDocument4 pagesPaperclip Activity-Dna Replicationapi-201291946No ratings yet
- Neurophysiology 2Document100 pagesNeurophysiology 2Tajamul MalikNo ratings yet
- Nitrogen CycleDocument15 pagesNitrogen CycleKenji AlbellarNo ratings yet
- 3BC Listening Warm-Up Audio TranscriptDocument1 page3BC Listening Warm-Up Audio TranscriptEmilio MorochoNo ratings yet
- Mammal - WDocument53 pagesMammal - WPaul MNo ratings yet
- 01 Cancer NoteDocument4 pages01 Cancer NoteDerrick liNo ratings yet
- Chapter 20 - Embryology of The Genitourinary Tract - Campbell - Walsh-Wein UROLOGY 12thDocument40 pagesChapter 20 - Embryology of The Genitourinary Tract - Campbell - Walsh-Wein UROLOGY 12thkrisnaNo ratings yet
- Mitosis Vs Meiosis PracticeDocument3 pagesMitosis Vs Meiosis Practiceapi-293001217No ratings yet
- Untitled DesignDocument11 pagesUntitled DesignJake UseNo ratings yet
- Plant Kingdom Edited 14Document8 pagesPlant Kingdom Edited 14Aayush MalikNo ratings yet
- PDF 33 PDFDocument107 pagesPDF 33 PDFTiruneh GANo ratings yet
- 2021-Review-Organoids and Organs-On-Chips Gut-Microbe InteractionsDocument12 pages2021-Review-Organoids and Organs-On-Chips Gut-Microbe InteractionsCristian Felipe Sandoval QuiñonezNo ratings yet
- Yoruba Reproduction BiologyDocument2 pagesYoruba Reproduction Biologyajibade ademolaNo ratings yet
- Bit5021 Biodiversity-And-conservation TH 1.0 40 Bit5021Document2 pagesBit5021 Biodiversity-And-conservation TH 1.0 40 Bit5021Aninda KanjilalNo ratings yet
- Principles of Clinical CytogeneticsDocument34 pagesPrinciples of Clinical CytogeneticsZainab Jamal SiddiquiNo ratings yet
- Introduction To Microbiology: Dr. Venkata Krishna B. (M.SC., NET., PH.D.)Document27 pagesIntroduction To Microbiology: Dr. Venkata Krishna B. (M.SC., NET., PH.D.)Peak LevelNo ratings yet