Professional Documents
Culture Documents
Directions For Use of The Hazard Analysis Form
Uploaded by
Thị Thanh Ngân NguyễnOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Directions For Use of The Hazard Analysis Form
Uploaded by
Thị Thanh Ngân NguyễnCopyright:
Available Formats
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle,
e.g. Bacon
Directions for Use of the Hazard Analysis Form
1. Make sure that every step shown on the Process Flow Diagram is
entered in the Hazard Analysis Form. Make sure that each step has
the same name and number in both the Process Flow Diagram and the
Hazard Analysis Form.
2. Check the three categories of hazard (Biological, Chemical, Physical)
shown for each step.
a. If you think a listed hazard is not reasonably likely to occur,
leave it in column 2 (Food Safety Hazard) and enter “No” in
column 3 (Reasonably likely to occur?). Then provide a reason in
column 4.
b. If you think there are no relevant hazards for a particular
category delete the listed hazard and write “none” in column 2,
write “No” in column 3, provide a reason in column 4 and cross
out any information in columns 5 and 6.
c. If you think that a relevant hazard should be added at a step,
describe the hazard in column 2 (Food Safety Hazard). Then
determine whether the hazard is reasonably likely to occur and
put the answer in column 3. Then provide, in column 4, a reason
for deciding whether or not the hazard is reasonably likely to
occur.
i. For example, following an SSOP, SOP, or approved
formulation may make a hazard unlikely to occur, or a
supplier may provide a letter of guarantee stating that
the hazard should not be present.
ii. On the other hand, a history of outbreaks or
contamination related to a hazard would mean that the
hazard IS reasonably likely to occur.
Columns 5 and 6 can be left blank if a hazard is NOT reasonably
likely to occur.
If the hazard IS reasonably likely to occur: fill in columns 5 and
6.
iii. In column 5, list measures that could be applied to
prevent, eliminate, or reduce the hazard to an acceptable
level. NOTE: at least one of these measures must be
either a Critical Control Point (CCP) at the present step,
or a CCP at a later step.
1 9/27/07 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle,
e.g. Bacon
iv. Finally, if the hazard is controlled by a CCP at the
present step, enter the CCP number in column 6. The
accepted numbering system is to number the CCP’s in
order, followed by either B, C, or P to indicate what type
of hazard is being controlled. For example, if the 2 nd CCP
in a process controlled a physical hazard, it would be
entered as CCP -2P.
d. I f you agree that a listed hazard is relevant, no changes are
necessary.
2 9/27/07 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
HAZARD ANALYSIS
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
1, Receiving and 6. Biological – No Visual inspection for
Storage – Packaging Contamination with container integrity,
materials meat, other biological contamination, at
materials receiving makes
hazard unlikely. SOP
for storage makes
hazard unlikely.
Chemical – Non-food No Letters of
grade materials; guarantee are
chemical contamination. received from all
suppliers of
packaging materials.
SOP for storage
makes hazard
unlikely.
Physical – None No SOPs for receiving
and storage make
hazard unlikely.
2. Receiving – Raw Biological - Presence No Raw meat/poultry is
Meat of unacceptably high (vegetative a known source of
levels of vegetative pathogens) pathogens, but
pathogens: receiving SOP makes
Salmonella, Listeria it unlikely that
monocytogenes, unacceptably high
3 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
Staphylococcus aureus. levels of pathogens
are present.
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Presence of Yes (spore- Raw meat is a known Hazard will be controlled by later
sporeforming forming source of pathogens. CCPs of smoking (prevents
pathogens: Clostridium pathogens) growth) and cooling (prevents
perfringens, germination and growth of
Clostridium botulinum. clostridial spores).
Chemical – None No SOP for receiving
makes hazard
unlikely.
Physical – None No SOP for receiving
makes hazard
unlikely.
4 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
3. Receiving – Food Biological- None No SOP for receiving
(Non-meat) makes hazard
Ingredients: spices, unlikely.
cure mix.
Chemical –Ingredients No letter of guarantee
containing undesirable is received from
substances each supplier of
food additives,
spices, cure mix,
etc.
Physical – None No SOP for receiving
makes hazard
unlikely.
4. Storage(Frozen/ Biological - Presence No Raw meat/poultry is
Refrigerated) – Raw of unacceptably high (vegetative a known source of
Meat levels of vegetative pathogens) pathogens, but
pathogens: receiving SOP makes
Salmonella, Listeria it unlikely that
monocytogenes, unacceptably high
Staphylococcus aureus. levels of pathogens
are present.
Product is labeled to
instruct consumers
to fully cook product
5 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
and thereby kill
pathogens.
Presence of spore- Yes (spore- Raw meat is a known Hazard will be controlled by later
forming pathogens: forming source of pathogens. CCPs of smoking (prevents
Clostridium pathogens) growth) and cooling (prevents
perfringens, germination and growth of
Clostridium botulinum. clostridial spores). Product is
labeled to instruct consumers to
fully cook product and thereby
kill pathogens.
Chemical - None No SOP for storage
makes hazard
unlikely.
Physical – None No SOP for storage
makes hazard
unlikely.
5. Storage – Food Biological – None No SOP for storage
Ingredients, both makes hazard
refrigerated and non- unlikely.
refrigerated Chemical – None No SOP for storage
makes hazard
unlikely.
Physical – None No SOP for storage
makes hazard
unlikely.
6 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
7. Tempering of raw Biological – Presence of No Raw meat/poultry is
meat unacceptably high (Presence of a known source of
levels of vegetative vegetative pathogens, but
pathogens: pathogens) receiving SOP makes
Salmonella, Listeria it unlikely that
monocytogenes, unacceptably high
Staphylococcus aureus. levels of pathogens
are present.
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Growth of vegetative No Pathogen growth is
pathogens. (Growth of unlikely because
vegetative tempering is done
pathogens) according to SOP
for
Tempering/Thawing
of Frozen Materials.
Presence of spore- Yes Raw meat is a known Hazard will be controlled by later
forming pathogens: (Presence of source of pathogens. CCPs of smoking (prevents
Clostridium pathogens) growth) and cooling (prevents
perfringens, germination and growth of
7 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
Clostridium botulinum. clostridial spores).
Growth of spore- No Pathogen growth is
forming pathogens. (Growth of unlikely because
spore- tempering is done
forming according to SOP
pathogens) for
Tempering/Thawing
of Frozen Materials.
Chemical – None No SSOP makes hazard
unlikely.
Physical - None No SSOP makes hazard
unlikely.
8. Preparing cure Biological – No Finished product
Insufficient nitrite color would be
level allowing growth of unacceptable if
spore-forming insufficient cure
pathogens added – product
would not enter
commerce
Chemical – Ingredients No Approved
not being added or (improper formulations are
used as intended; use) followed.
8 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
cleaning/sanitizing No Pre-op SSOP makes
chemical residues; (cleaning presence of
chemicals) chemical residues
unlikely to occur.
cure-mix may contain No Application of
potential allergens; (allergens) correct label
prevents
inadvertent
consumption of
allergen by
consumer.
Operational SSOP
prevents cross-
contamination of
allergenic agents.
excess cure addition No Cure mix has highly
(excess cure) dilute concentration
of nitrite; amount
used is logged on
batch sheet.
Physical - None No SSOP makes hazard
unlikely.
9. Dry-rubbing Biological – Presence of No Raw meat/poultry is
9 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
unacceptably high (Presence of a known source of
levels of vegetative vegetative pathogens, but
pathogens (see list in pathogens) receiving SOP makes
step 2 above); it unlikely that
unacceptably high
levels of pathogens
are present.
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Pathogen contamination No SSOP makes hazard
via equipment (Contamina- unlikely.
(contaminated at start tion)
of shift) and workers;
Presence of spore- Yes Raw meat is a known Hazard will be controlled by later
forming pathogens. (presence of source of pathogens. CCPs of smoking (prevents
spore- growth) and cooling (prevents
forming germination and growth of
pathogens) clostridial spores).
10 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
Insufficient nitrite No Finished product
level allowing growth of (Growth of color would be
spore-forming spore- unacceptable if
pathogens forming insufficient cure
pathogens) added – product
would not enter
commerce
Growth of spore- No (Growth Duration of step is
formation pathogens of spore- short enough that
before cure acts forming growth would not
pathogens) occur.
Chemical – No Pre-op SSOP makes
cleaning/sanitizing (cleaning presence of
chemical residues; chemicals) chemical residues
excess cure addition unlikely to occur.
No Cure mix has highly
(excess cure) dilute concentration
11 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
of nitrite; amount
used is logged on
batch sheet.
Physical – None No SSOP makes hazard
unlikely
10. Pumping Biological – Presence of No Raw meat/poultry is
unacceptably high (Presence of a known source of
levels of vegetative vegetative pathogens, but
pathogens (see list in pathogens) receiving SOP makes
step 2 above); it unlikely that
unacceptably high
levels of pathogens
are present.
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Pathogen contamination No SSOP makes hazard
via equipment (Contamina- unlikely.
(contaminated at start tion)
of shift) and workers;
12 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
Presence of spore- Yes Raw meat is a known Hazard will be controlled by later
forming pathogens. (presence of source of pathogens. CCPs of smoking (prevents
spore- growth) and cooling (prevents
forming germination and growth of
pathogens) clostridial spores).
Insufficient nitrite No Finished product
level allowing growth of (Growth of color would be
spore-forming spore- unacceptable if
pathogens forming insufficient cure
pathogens) added – product
would not enter
commerce
Growth of spore- No (Growth Duration of step is
forming pathogens of spore- short enough that
before cure acts forming growth would not
pathogens) occur.
Chemical – Ingredients No Application of
may contain potential (allergens) correct label
allergens; prevents
13 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
cleaning/sanitizing inadvertent
chemical residues; consumption of
excess cure addition allergen by
consumer.
Operational SSOP
prevents cross-
contamination of
allergenic agents.
No Pre-op SSOP makes
(cleaning presence of
chemicals) chemical residues
unlikely to occur.
No Cure mix has highly
(excess cure) dilute concentration
of nitrite; amount
used is logged on
batch sheet.
Physical – None No SSOP makes hazard
unlikely.
11. Injecting Biological – Presence of No Raw meat/poultry is
unacceptably high (Presence of a known source of
levels of vegetative vegetative pathogens, but
pathogens (see list in pathogens) receiving SOP makes
step 2 above); it unlikely that
14 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
unacceptably high
levels of pathogens
are present.
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Pathogen contamination No SSOP makes hazard
via equipment (Contamina- unlikely.
(contaminated at start tion)
of shift) and workers;
Presence of spore- Yes Raw meat is a known Hazard will be controlled by later
forming pathogens. (presence of source of pathogens. CCPs of smoking (prevents
spore- growth) and cooling (prevents
forming germination and growth of
pathogens) clostridial spores).
Insufficient nitrite No Finished product
level allowing growth of (Growth of color would be
spore-forming spore- unacceptable if
pathogens forming insufficient cure
15 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
pathogens) added – product
would not enter
commerce
Growth of spore- No (Growth Duration of step is
forming pathogens of spore- short enough that
before cure acts. forming growth would not
pathogens) occur.
Chemical – Ingredients No Application of
may contain potential (allergens) correct label
allergens; prevents
cleaning/sanitizing inadvertent
chemical residues; consumption of
excess cure addition allergen by
consumer.
Operational SSOP
prevents cross-
contamination of
allergenic agents.
16 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
No Pre-op SSOP makes
(cleaning presence of
chemicals) chemical residues
unlikely to occur.
No Cure mix has highly
(excess cure) dilute concentration
of nitrite; amount
used is logged on
batch sheet.
Physical – Metal from No No history of
injector problem (must
provide evidence).
Visual inspection of
equipment during
cleaning makes it
unlikely that hazard
enters commerce.
12. Tumbling Biological – Presence of No Raw meat/poultry is
unacceptably high (Presence of a known source of
levels of vegetative vegetative pathogens, but
pathogens (see list in pathogens) receiving SOP makes
step 2 above); it unlikely that
unacceptably high
levels of pathogens
17 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
are present.
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Pathogen contamination No SSOP makes hazard
via equipment (Contamina- unlikely.
(contaminated at start tion)
of shift) and workers;
Presence of spore- Yes Raw meat is a known Hazard will be controlled by later
forming pathogens. (presence of source of pathogens. CCPs of smoking (prevents
spore- growth) and cooling (prevents
forming germination and growth of
pathogens) clostridial spores).
Insufficient nitrite No Finished product
level allowing growth of (Growth of color would be
spore-forming spore- unacceptable if
pathogens forming insufficient cure
pathogens) added – product
would not enter
18 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
commerce
Growth of spore- No (Growth Duration of step is
forming pathogens of spore- short enough that
before cure acts. forming growth would not
pathogens) occur.
Chemical – No Pre-op SSOP makes
cleaning/sanitizing presence of
chemical residues chemical residues
unlikely to occur.
Physical - Metal No No history of
problem (must
provide evidence).
Visual observation
for foreign
materials during
processing,
inspection of
equipment during
cleaning make
hazard unlikely.
19 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
13. Brining Biological – Presence of No Raw meat/poultry is
unacceptably high (Presence of a known source of
levels of vegetative vegetative pathogens, but
pathogens (see list in pathogens) receiving SOP makes
step 2 above); it unlikely that
unacceptably high
levels of pathogens
are present.
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Pathogen contamination No SSOP makes hazard
via equipment (Contamina- unlikely.
(contaminated at start tion)
of shift) and workers;
Presence of spore- Yes Raw meat is a known Hazard will be controlled by later
forming pathogens. (presence of source of pathogens. CCPs of smoking (prevents
spore- growth) and cooling (prevents
forming germination and growth of
pathogens) clostridial spores).
20 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
Insufficient nitrite No Finished product
level allowing growth of (Growth of color would be
spore-forming spore- unacceptable if
pathogens forming insufficient cure
pathogens) added – product
would not enter
commerce
Growth of spore- No (Growth Step is done under
forming pathogens of spore- refrigeration to
forming prevent growth.
pathogens)
Chemical – No Pre-op SSOP makes
cleaning/sanitizing presence of
chemical residues chemical residues
unlikely to occur.
Physical - None No SSOP makes hazard
unlikely.
21 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
14.Hanging in cooler Biological – Presence of No Raw meat/poultry is
unacceptably high (Presence of a known source of
levels of vegetative vegetative pathogens, but
pathogens (see list in pathogens) receiving SOP makes
step 2 above); it unlikely that
unacceptably high
levels of pathogens
are present.
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Pathogen contamination No SSOP makes hazard
via equipment (Contamina- unlikely.
(contaminated at start tion)
of shift) and workers;
Presence of spore- Yes Raw meat is a known Hazard will be controlled by later
forming pathogens. (presence of source of pathogens. CCPs of smoking (prevents
spore- growth) and cooling (prevents
forming germination and growth of
pathogens) clostridial spores).
22 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
Growth of vegetative No Cooler temperature
or spore-forming (Growth of is maintained cold
pathogens spore- enough to prevent
forming pathogen growth,
pathogens) per SOP for
storage.
Chemical - None No SSOP makes hazard
unlikely.
Physical - None No SSOP makes hazard
unlikely.
15. smoking Biological – Presence of No Raw meat/poultry is
unacceptably high (Presence of a known source of
levels of vegetative vegetative pathogens, but
pathogens (see list in pathogens) receiving SOP makes
step 2 above); it unlikely that
unacceptably high
levels of pathogens
are present.
23 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
Product is labeled to
instruct consumers
to fully cook product
and thereby kill
pathogens.
Presence of spore- Yes Raw meat is a known Hazard will be controlled by this
forming pathogens. (presence of source of pathogens. CCP of smoking (prevents growth)
spore- and the later CCP of cooling
forming (prevents germination and growth
pathogens) of clostridial spores).
Growth of spore- No Presence of nitrite,
forming pathogens (Growth of relatively short
spore- duration, and
forming relatively low
pathogens) temperature make
germination of
Production of heat- Yes spores unlikely. CCP 1-B
stable enterotoxin by Product is labeled to This step is a CCP. Time and
S. aureus instruct consumers temperature of smoking will be
to fully cook product controlled to prevent S. aureus
and thereby kill growth.
germinated cells.
S. aureus is salt-
24 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
tolerant and forms a
toxin that may not
be destroyed during
cooking.
Chemical – None No SSOP makes hazard
unlikely.
Physical - None No SSOP makes hazard
unlikely.
16. Cooling Biological – growth of Yes Spores survive This step is a CCP. Product is CCP 2-B
Clostridium (clostridial cooking and can cooled rapidly enough to prevent
perfringens or growth) germinate and grow growth of Clostridium
Clostridium botulinum; if cooling is done too perfringens (> 1 log) or
slowly. Clostridium botulinum.
Recontamination of No Pre-op and
product with pathogens (Recontamina Operational SSOPs
tion) make contamination
unlikely.
No Product is
Growth of (Growth of refrigerated or
25 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
recontaminating L.m. or other frozen (minimizes or
pathogens. pathogens) prevents pathogen
growth)
Chemical – None No
Physical - None No
17. Slicing and 18. Biological - Growth of No Product does not
Packaging Clostridium (clostridial get warm enough for
perfringens or growth) a long enough time
Clostridium botulinum; during slicing and
packaging to result
in growth.
Recontamination with No Pre-op and
pathogens; (Presence of operational SSOP
L.m. or other make contamination
pathogens) unlikely.
Growth of No Duration of slicing is
recontaminating (Growth of short enough to
pathogens. L.m. or other prevent pathogen
pathogens) growth. Product is
kept
refrigerated/frozen
(minimizes/prevents
pathogen growth)
Chemical – improperly No Labeling SOP and
26 05/11/2012 version; supersedes all previous versions
Heat Treated, But Not Fully Cooked - not Shelf-Stable: cured whole muscle, e.g. Bacon
1. Process Step 2. Food Safety 3. 4. Basis of 5. If Yes in Column 3, 6. Critical
Hazard Reasonably Reasonably What Measures Could be Control Point
likely to likely to occur Applied to Prevent,
occur Eliminate, or Reduce the
Hazard to an Acceptable
Level?
labeled allergens SSOP make hazard
unlikely.
Physical - metal No No history of
problem (must
provide evidence).
Visual inspection of
equipment during
cleaning makes it
unlikely that hazard
enters commerce.
19. Shipping or retail Biological - None No SSOP makes hazard
sale unlikely.
Chemical - None No SSOP makes hazard
unlikely.
Physical - None No SSOP makes hazard
unlikely.
27 05/11/2012 version; supersedes all previous versions
You might also like
- Drug Testing in Child WelfareDocument49 pagesDrug Testing in Child WelfareLOLA100% (1)
- Operator Handbook Red Team Osint Blue Team Reference V10nbsped 9798605493952Document443 pagesOperator Handbook Red Team Osint Blue Team Reference V10nbsped 9798605493952harshamlNo ratings yet
- The 5 Fundamental of Fat LossDocument16 pagesThe 5 Fundamental of Fat LossAndrés Jimenez100% (2)
- Azard Nalysis and Ritical Ontrol Oint: H A C C PDocument47 pagesAzard Nalysis and Ritical Ontrol Oint: H A C C PFranz MarasiganNo ratings yet
- The Body Remembers Volume 2: Revolutionizing Trauma Treatment - Babette RothschildDocument6 pagesThe Body Remembers Volume 2: Revolutionizing Trauma Treatment - Babette Rothschildnamonomi0% (4)
- International General Certificate Assessor's Marking Sheet (2011 Specification) Igc3 - The Health and Safety Practical ApplicationDocument11 pagesInternational General Certificate Assessor's Marking Sheet (2011 Specification) Igc3 - The Health and Safety Practical ApplicationVigneshwaraNo ratings yet
- Receiving ingredients HACCP analysisDocument26 pagesReceiving ingredients HACCP analysisAYMAN ZEHRANo ratings yet
- Food Safety Management Plan SummaryDocument3 pagesFood Safety Management Plan Summarysafety officer100% (2)
- Submitted By: Lantape, Ryan Luis P. RISK 111 Balandang, Jymel Gabriel Submitted To: Mrs. Mylene Dela Cruz Palmares, Vincent RileyDocument3 pagesSubmitted By: Lantape, Ryan Luis P. RISK 111 Balandang, Jymel Gabriel Submitted To: Mrs. Mylene Dela Cruz Palmares, Vincent RileyJhon Rommel Dela Cruz-OdiamanNo ratings yet
- Manufacturing Steps of Butter ChickenDocument27 pagesManufacturing Steps of Butter ChickenAYMAN ZEHRA75% (4)
- HACCP Plan Template FermentationDocument18 pagesHACCP Plan Template FermentationAugustina AnaglateyNo ratings yet
- Summary MiasmsDocument8 pagesSummary MiasmsPranesh PeterNo ratings yet
- Lesson 2 Risk Management As Applied To Safety, SecurityDocument30 pagesLesson 2 Risk Management As Applied To Safety, SecurityMarlon AndayaNo ratings yet
- HACCP Plan for Raw Ground PizzaDocument13 pagesHACCP Plan for Raw Ground PizzaLe DioNo ratings yet
- Hazard Analysis Worksheet (Apple Juice)Document8 pagesHazard Analysis Worksheet (Apple Juice)JyShe_la100% (1)
- Pizza HaccpDocument13 pagesPizza HaccpLe Dio100% (2)
- Reading Final Qs 1 1 PDFDocument64 pagesReading Final Qs 1 1 PDFMuntaserAlawiNo ratings yet
- Transes MLSP-112Document60 pagesTranses MLSP-112Hermione GrangerNo ratings yet
- HACCP Principles Comparison TableDocument27 pagesHACCP Principles Comparison TablefushiersNo ratings yet
- HACCP Plan Template Sample ReportDocument7 pagesHACCP Plan Template Sample ReportgustasalNo ratings yet
- SITXFSA008 Food Safety Program TemplateDocument26 pagesSITXFSA008 Food Safety Program Templateshanmugam thevapriyan50% (2)
- FDC Organic Virgin Coconut Oil Hazard Analysis and Critical Control Points Plan PDFDocument3 pagesFDC Organic Virgin Coconut Oil Hazard Analysis and Critical Control Points Plan PDFWynona Basilio100% (2)
- Yogurt HACCP Plan PDFDocument38 pagesYogurt HACCP Plan PDFserenela100% (2)
- Intake and Output CalculationDocument20 pagesIntake and Output CalculationJaycee Silva100% (2)
- CSS - Hazards and RisksDocument1 pageCSS - Hazards and RisksRowell MarquinaNo ratings yet
- HACCP Sheep Goat Pork SlaughterDocument19 pagesHACCP Sheep Goat Pork SlaughterFatou Sarr100% (1)
- Essential Practices for Managing Chemical Reactivity HazardsFrom EverandEssential Practices for Managing Chemical Reactivity HazardsNo ratings yet
- Critical Control Points of Fish Longganisa ProductionDocument2 pagesCritical Control Points of Fish Longganisa Productionjezu roqNo ratings yet
- Food Safety Handbook - Part 2Document203 pagesFood Safety Handbook - Part 2Yasser MostafaNo ratings yet
- Python Cheat SheetDocument16 pagesPython Cheat Sheetphamxtien3741No ratings yet
- LC HAGroundBeefDocument2 pagesLC HAGroundBeefAnar MahmudovNo ratings yet
- Hazard Analysis Template v.2019.02.13Document2 pagesHazard Analysis Template v.2019.02.13amir ShehzadNo ratings yet
- NCP Pt. DE ASISDocument3 pagesNCP Pt. DE ASISRhea Mae Valles - ReyesNo ratings yet
- Hazard Analysis ChartDocument9 pagesHazard Analysis Chartapi-410362945No ratings yet
- RTE Hazard Analysis Review of Hot Dogs Identifies Cooking and Cooling as CCPsDocument8 pagesRTE Hazard Analysis Review of Hot Dogs Identifies Cooking and Cooling as CCPsStanislava KanjevacNo ratings yet
- ISO 22000 food safety fundamentalsDocument14 pagesISO 22000 food safety fundamentalsVineeth Kumar MishraNo ratings yet
- Bio Risk Part 1Document4 pagesBio Risk Part 1CXT EnterpriseNo ratings yet
- 07-Hazard Analysis, 007Document4 pages07-Hazard Analysis, 007sajid waqasNo ratings yet
- Biorisk Mitigation Strategies - PASMETH - PhBBA - DTRADocument66 pagesBiorisk Mitigation Strategies - PASMETH - PhBBA - DTRARyan Marañon PedregosaNo ratings yet
- SushiDocument27 pagesSushishani31No ratings yet
- 17a IM Est Process Hazard AnalysisDocument27 pages17a IM Est Process Hazard AnalysisNader SedighiNo ratings yet
- HACCP ImplementationDocument25 pagesHACCP ImplementationAronn SiamNo ratings yet
- Hazard Analysis & HACCP WorksheetsDocument3 pagesHazard Analysis & HACCP WorksheetsJohn Cedric DullaNo ratings yet
- Antimicrobial Susceptibility Test ResultsDocument7 pagesAntimicrobial Susceptibility Test Resultsangela roperezNo ratings yet
- BMS533 Practical 1Document10 pagesBMS533 Practical 1NURUL AIHAN AHMAD HILMINo ratings yet
- Lunchbox HACCP PlanDocument18 pagesLunchbox HACCP PlanFooTraGoNo ratings yet
- Hazard Analysis and Critical Control Point (Haccp)Document7 pagesHazard Analysis and Critical Control Point (Haccp)Elsadig AmnconsNo ratings yet
- Pembetulan Peta Minda SlideDocument3 pagesPembetulan Peta Minda SlideNur Husnina ZawawiNo ratings yet
- Indonesian Symposium Looks at The ABC of The Biosecurity ChallengeDocument2 pagesIndonesian Symposium Looks at The ABC of The Biosecurity ChallengeDrivailaNo ratings yet
- Managing Biosafety and Biosecurity Risks in Veterinary LabsDocument13 pagesManaging Biosafety and Biosecurity Risks in Veterinary LabsallatkertNo ratings yet
- Guidance For Environmental Sampling ProgramDocument4 pagesGuidance For Environmental Sampling Programalias brownNo ratings yet
- Haccp WsDocument11 pagesHaccp WsFreg GregNo ratings yet
- Feed Safety Basics For VeterinariansDocument4 pagesFeed Safety Basics For VeterinariansŠhâh NawazNo ratings yet
- 6 Ways To Prevent Food ContaminationDocument1 page6 Ways To Prevent Food ContaminationKriszzia Janilla ArriesgadoNo ratings yet
- Conduct a hazard analysis for food safetyDocument5 pagesConduct a hazard analysis for food safetyjujuNo ratings yet
- Taking Salmonella ProblemDocument4 pagesTaking Salmonella ProblemM.C. Ángel ArteagaNo ratings yet
- Safety Security Biosafety Laboratory Biosecurity PerspectiveDocument5 pagesSafety Security Biosafety Laboratory Biosecurity PerspectiveZabdiel Ann SavellanoNo ratings yet
- 4th Module 1Document18 pages4th Module 1Mha RizNo ratings yet
- HACCP Exercise Assignment Wheat Flour MatterhornDocument10 pagesHACCP Exercise Assignment Wheat Flour MatterhornZigiju ZelekeNo ratings yet
- Laboratory Biosafety and Biosecurity ConceptsDocument6 pagesLaboratory Biosafety and Biosecurity ConceptsCXT EnterpriseNo ratings yet
- Work Activity Inventory Sheet Word TemplateDocument7 pagesWork Activity Inventory Sheet Word TemplateHendrawan ChandraNo ratings yet
- Shrimp DriedDocument5 pagesShrimp DriedbinchooknationNo ratings yet
- Manage food safety with HACCPDocument10 pagesManage food safety with HACCPCamille AlberichNo ratings yet
- Risk Managemnt ReviewerDocument4 pagesRisk Managemnt ReviewerHaru CutieNo ratings yet
- BMS533 Lab Practical 1Document13 pagesBMS533 Lab Practical 1Nur NazirahNo ratings yet
- Risk ManagementDocument5 pagesRisk ManagementTrisha Anne MorionesNo ratings yet
- Icheme Symposium Series No. 124Document8 pagesIcheme Symposium Series No. 124Amarnath Reddy RagipindiNo ratings yet
- CXS 192eDocument490 pagesCXS 192eRosita handrianaNo ratings yet
- Appetite: Jordan E. Lyerly, Charlie L. ReeveDocument9 pagesAppetite: Jordan E. Lyerly, Charlie L. ReeveHanh PhamNo ratings yet
- Willingness To Try InsectsDocument58 pagesWillingness To Try InsectsThị Thanh Ngân NguyễnNo ratings yet
- Guerrero2010 PDFDocument9 pagesGuerrero2010 PDFRomi Lycantrophe DieorliveNo ratings yet
- Guerrero2009 PDFDocument10 pagesGuerrero2009 PDFRomi Lycantrophe DieorliveNo ratings yet
- Nutrients 09 00088Document17 pagesNutrients 09 00088Familia Uribe-fuentesNo ratings yet
- Association between traditional food consumption and motivesDocument8 pagesAssociation between traditional food consumption and motivesdaneunbiNo ratings yet
- Guerrero2010 PDFDocument9 pagesGuerrero2010 PDFRomi Lycantrophe DieorliveNo ratings yet
- Willingness To Try InsectsDocument58 pagesWillingness To Try InsectsThị Thanh Ngân NguyễnNo ratings yet
- RCP y PH ArterialDocument2 pagesRCP y PH ArterialPaola LizarzabalNo ratings yet
- Putnam Voice - 1/11/12Document10 pagesPutnam Voice - 1/11/12The Lima NewsNo ratings yet
- Research EthicsDocument2 pagesResearch EthicsCarla HAHAHAHANo ratings yet
- Soal Kuis 7 Ppu - Bahasa Inggris: Text 1Document3 pagesSoal Kuis 7 Ppu - Bahasa Inggris: Text 1rayhan taufikNo ratings yet
- Date: 9/10/15 Week of 9/07/15Document4 pagesDate: 9/10/15 Week of 9/07/15api-296922357No ratings yet
- Is It Better To Do Cardio Before or After Weight Training?Document4 pagesIs It Better To Do Cardio Before or After Weight Training?Radhakrishnan SreerekhaNo ratings yet
- Marketing challenges at St. Margaret's HospitalDocument2 pagesMarketing challenges at St. Margaret's HospitalMihaela Papusica0% (1)
- Ignatavicius TOCDocument9 pagesIgnatavicius TOCjennaaahhhNo ratings yet
- Product Safety Data Sheet: Moringa Body LotionDocument2 pagesProduct Safety Data Sheet: Moringa Body LotionemyNo ratings yet
- LeprosyDocument22 pagesLeprosyvisweswar030406No ratings yet
- Professional Services, Inc. vs. Natividad and AganaDocument2 pagesProfessional Services, Inc. vs. Natividad and AganaBryan Albert SuerteNo ratings yet
- AMDLI COVID-19 Test ResultDocument2 pagesAMDLI COVID-19 Test ResultMarco Dela CruzNo ratings yet
- Intensivist To Patient RatioDocument8 pagesIntensivist To Patient RatioroykelumendekNo ratings yet
- NetworkList (NAS)Document70 pagesNetworkList (NAS)Fahad MohammadNo ratings yet
- Perioperative Nursing Cesarean SectionDocument13 pagesPerioperative Nursing Cesarean Sectionmark OrpillaNo ratings yet
- Department of Education: Republic of The PhilippinesDocument4 pagesDepartment of Education: Republic of The PhilippinesJESSIELYNE LA ROSANo ratings yet
- Main Match Results and Data 2017Document124 pagesMain Match Results and Data 2017Tǻɲ ṦħǻNo ratings yet
- Documentation in Colorectal and Stoma Care Nursing RCN 2013Document24 pagesDocumentation in Colorectal and Stoma Care Nursing RCN 2013Bhie BhieNo ratings yet
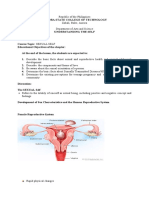
- Module 6 - Sexual SelfDocument14 pagesModule 6 - Sexual SelfFlorence de LeonNo ratings yet
- Kidney Treatment Guidelines For HyponatremiaDocument7 pagesKidney Treatment Guidelines For HyponatremialguerreroNo ratings yet
- Sample Question PaperDocument3 pagesSample Question Paperamar_deshmukh713710No ratings yet
- 3M 9105 Wear It Right Poster PDFDocument2 pages3M 9105 Wear It Right Poster PDFScott KramerNo ratings yet