Professional Documents
Culture Documents
Thrombosis in Pregnancy Review 1999
Uploaded by
georgianaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thrombosis in Pregnancy Review 1999
Uploaded by
georgianaCopyright:
Available Formats
Thrombosis
Thrombosis in pregnancy: maternal and fetal issues
Ian A Greer
Pulmonary thromboembolism is the main cause of maternal death in the UK and current trends show an increase.
Deep-vein thrombosis underlies this disorder. Important issues include pathophysiology, diagnosis, and management
of thrombosis in pregnancy, especially the use of anticoagulants. Congenital and acquired thrombophilias contribute
to the pathophysiological processes that underlie miscarriage, intrauterine growth restriction, and pre-eclampsia, and
raises new possibilities for intervention. The high prevalence of thrombophilic defects in the population, the
association of defects with maternal and fetal disorders, and special considerations for management make it
essential for obstetricians to understand this area.
In the UK, pulmonary thromboembolism is the leading Other factors that underlie venous thromboembolism
cause of maternal death.1 Deep-vein thrombosis (DVT) in pregnancy include weight over 80 kg, family or
underlies this disorder and is frequently unrecognised.2 personal history of thrombosis, and thrombophilia.8
DVT in pregnancy is associated with an increased risk Venous thromboembolism during pregnancy is associated
of further thrombosis and deep-vein insufficiency. Risk with an increased risk of future venous
factors for DVT include congenital and acquired thromboembolism. Almost two-thirds of women with
thrombophilias, which probably play a part in the venous thromboembolism during pregnancy develop
pathogenesis of miscarriage, intrauterine growth deep-vein insufficiency in the affected leg,9 a rate
restriction, and pre-eclampsia. Perhaps the most substantially higher than would be expected after DVT
compelling evidence is the association between thrombo- outside of pregnancy. This increase may reflect the higher
philia due to antiphospholipid antibodies and the incidence of iliofemoral vein thrombosis during
activation of the coagulation-system3 and placental pregnancy.
infarction. A randomised controlled trial4 has shown that
aspirin and heparin given to reduce coagulation activity Pathogenesis of venous thromboembolism
improved the outcome of pregnancy. The role of Virchow’s triad of underlying factors in venous
anticardiolipin antibody syndrome in such complications thrombosis—hypercoagulability, venous stasis, and
is explored later in this series by Michael Greaves.5 This vascular damage—all occur in pregnancy. During
paper focuses on maternal and fetal issues associated with pregnancy there are increases in procoagulant factors,
venous thrombosis and congenital thrombophilias. such as von Willebrand factor, factor VIII, factor V,
and fibrinogen, that occur together with an acquired
Epidemiology of maternal venous resistance to the endogenous anticogulant, activated
thromboembolism protein C, and a reduction in protein S, the co-factor for
protein C. 10 These changes are accompanied by impaired
The overall incidence of fatal pulmonary thrombo-
fibrinolysis through increases in plasminogen activator
embolism associated with pregnancy has fallen since the
inhibitors 1 and 2, the latter being produced by the
early 1950s, especially among women after vaginal
placenta.11 These changes represent the physiological
delivery (figure 1). However, since the 1980s, this downward
preparation for the haemostatic challenge of delivery.
trend has been reversed,1 which highlights the need for
Venous stasis occurs in pregnancy by the end of the first
thromboprophylaxis after vaginal delivery in all women at
trimester and reaches a nadir at 36 weeks.12 Endothelial
risk of thromboembolism. The rate of non-fatal events is damage to pelvic vessels can occur during vaginal or
more difficult to establish than that for fatal ones. The abdominal delivery. Thus, the scene is set for the
incidence of antenatal DVT is about 0·615 per 1000 development of thrombosis in pregnancy.
pregnancies in women aged younger than 35 years and Almost 90% of DVT affect the left side among
1·216 per 1000 in women older than 35 years.6 The rate pregnant women compared with 55% among women
of postpartum DVT is about 0·304 per 1000 pregnancies who are not pregnant.8,9 This difference may reflect
in women younger than 35 years and 0·72 per 1000 in compression of the left iliac vein by the right iliac and the
women older than 35 years. Antenatal DVT is more ovarian arteries which cross the vein on the left side only.
common than postpartum DVT,6,7 for which data may Furthermore, in pregnancy most cases of DVT are
not be accurate and complete because women may ileofemoral rather than calf vein thrombosis (72% vs 9%),
present to internal medicine rather than to obstetric and iliofemoral DVT is more likely than calf-vein
services. Almost 40% of postpartum DVT manifests after thrombosis to lead to pulmonary thromboembolism.
discharge from hospital. Age and operative delivery are DVT in pregnancy can present, or be associated with,
major risk factors for venous thromboembolism (figure 2). lower abdominal pain due to periovarian collateral
circulation or thrombosis. When coupled with the mild
Lancet 1999; 353: 1258–65 pyrexia and leucocytosis of venous thromboembolism,
Department of Obstetrics and Gynaecology, University of Glasgow, this pain can be mistaken for other intra-abdominal
Glasgow Royal Infirmary, Glasgow G31 2ER, UK (Prof I A Greer MD) disorders, such as urinary tract infection or appendicitis.
1258 THE LANCET • Vol 353 • April 10, 1999
during pregnancy among women with
thrombophilia in the absence of anti-
coagulant therapy was initially judged to be
about 60% among pregnant women
with antithrombin deficiency.14,20 However,
retrospective studies20,23 of symptomatic
kindred showed the incidence of venous
thromboembolism among these women to
be 32% to 44%. For pregnant women with
abnormalities of the protein C and protein
S system the risk is substantially lower than
for antithrombin-deficient women, and
postpartum thromboses are more common
than antepartum thrombosis. The risk of
thrombosis in pregnancy is 3–10% for
protein C deficiency and 0–6% for protein
S deficiency. In postpartum women the
risk is 7–19% for protein C deficiency and
7–22% for protein S deficiency.20–23
Activated protein C resistance, this defect
was found in up to 78% of women
investigated for venous thrombosis in
pregnancy,24 whereas the factor V Leiden
genotype was found in up to 46%.25 These
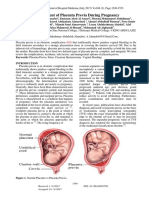
Figure 1: Number of cases of fatal pulmonary thromboembolism in pregnancy studies, however, investigated women with
and the puerperium in England 1955–84, UK 1985–96 venous thromboembolism and provide
Data from Confidential Enquiries into Maternal Deaths. limited information about the risk of
thrombosis in previously symptom-free
Congenital thrombophilia and maternal venous women with the mutation. In a study of 43 women with
thromboembolism the factor V Leiden mutation from symptomatic families,
The thrombophilias, which were discussed by Fritz the overall incidence of pregnancy-associated thrombosis
Rosendaal13 earlier in this series, underlie many was 14%.22 This risk may be higher postpartum.26
thrombotic disorders in pregnancy. 14 The main congenital Thus, pregnancy seems to be a significant factor that can
precipitate venous thromboembolic complications in
thrombophilias are deficiencies of antithrombin, protein
women with this mutation. However, these studies
C, and protein S, and the presence of factor V Leiden,
assess symptomatic kindred and so may overestimate the
the prothrombin gene variant, and homozygosity for
risk.
the thermolabile variant of methylenetetrahydrofolate
In McColl and colleagues’ 1997 retrospective study8 of
reductase (MTHFR). Factor V Leiden manifests as
about 72 000 pregnancies, pregnant women with venous
resistance to activated protein C and is the most common
thromboembolism were screened for thrombophilia. The
defect underlying venous thrombembolism, but such 16
underlying prevalence of congenital thrombophilia in
resistance can be caused by disorders other than factor V this population had already been established and the
Leiden, including antiphospholipid antibody syndrome investigators estimated the risk of venous thrombo-
and other genetic defects in the factor V molecule. The embolism in pregnancy to be 1 in 437 for factor V
resistance can also be acquired in pregnancy as a result of Leiden, 1 in 113 for protein C deficiency, 1 in 2·8 for
increases in factor V and factor VIII.10,16 type I (quantitative) antithrombin deficiency, and 1 in 42
17
The genetic variation in the prothrombin gene has
been linked to venous thromboembolism in pregnancy.18
However, more information is needed about this defect
in pregnancy, particularly because it may be found with
other inherited thrombophilias such as factor V Leiden.
Hyperhomocysteinaemia is an established risk factor for
venous and arterial thrombosis, and is most commonly
caused by homozygosity for the thermolabile variant
of MTHFR.19 Pregnancy is associated with decreased
concentrations of homocysteine and folic acid
supplements will also lower homocysteine concentrations.
However, the contribution of homocysteine to venous
thromboembolism in pregnancy is unclear.
The risk of maternal venous thromboembolism with
underlying thrombophilia will depend on the underlying
thrombophilic defect(s), history of thrombotic events,
and additional risk factors. As Rosendaal13 has pointed
out, clinical thrombosis is now considered a multicausal Figure 2: Incidence of postpartum deep-vein thrombosis
disease, resulting from interaction between congenital and pulmonary thromboembolism by maternal age and
and acquired risk factors. The risk of thromboembolism method of delivery
THE LANCET • Vol 353 • April 10, 1999 1259
Study and study design Study population Prevalence of factor V Leiden, activated protein C resistance, or both
protein C resistance, or both
Cases Controls
Rai et al 276 Cases: women with >3 miscarriages (a) 4/70 (5·7%) 3/70 (4·3%)
Case-control to assess prevalence (a) confined to first trimester (b) 10/50 (20%)*
of activated protein C resistance (b) including at least one second
trimester miscarriage
Controls: parous women with no history
of miscarriage
Grandone et al28 Cases: women with >2 miscarriages 7/42 (16·3%)† 5/118 (4·2%)
Case-control to assess prevalence Controls: parous women with no history
of factor V Leiden of miscarriage
Dizon-Townson et al30 Cases: women with spontaneous 12/176 (6·8%) 17/403 (4·2%)
Case-control to assess prevalence miscarriage
of factor V Leiden Controls: unselected pregnancies
Dizon-Townson et al32 Cases: women with >3 miscarriages 0/40 0/25
Case-control to assess prevalence Controls: parous women
of factor V Leiden
Belasch et al31 Cases: women with >2 consecutive 1/50 (2%) 1/50 (2%)
Case-control to assess prevalence first-trimester miscarriages
of factor V Leiden Controls: parous women with no history
of miscarriage
Brenner et al29 Cases: women with >3 consecutive first 19/39 (48%)‡ Not available
Cohort to assess activated protein or second-trimester miscarriages
C resistance and factor V Leiden
*p<0·02 versus controls and first-trimester miscarriage group.
†p<0·02 versus controls; cases were also more likely to have second-trimester loss (p<0·05).
‡An additional 5/39 had activated protein C resistance without factor V Leiden.
Table 1: Miscarriage, activated protein C resistance, and factor V Leiden
for type II (qualitative) antithrombin deficiency. There is aborted fetuses carried the genotype for factor V. This
a pressing need for more information on the natural finding accords with the higher frequency of placental
history of these disorders in pregnancy so that infarction in pregnancies associated with maternal and
obstetricians can assess risk and provide appropriate fetal carriage of factor V Leiden.30,33
thromboprophylaxis. Because the risk in symptom-free These case-control and cohort studies focused on
women with a familial thrombophilic trait, excluding women with a history of miscarriage. Taking another
antithrombin deficiency, is uncertain in pregnancy, a approach, Preston and colleagues34 investigated
randomised placebo-controlled trial of thrombopro- pregnancy outcome in a cohort of women with
phylaxis is justified to guide practice. thrombophilia. Compared with data from a healthy
control population, there was an increased risk of fetal
Congenital thrombophilia and fetal loss loss (odds ratio 1·35 [95% CI 1·01–1·82]) and stillbirth
The association between acquired resistance to activated (3·6 [1·4–9·4]), however, the risk of miscarriage was not
protein C and disorders such as anticardiolipin antibody significantly increased (1·27 [0·94–1·71]) (table 2). The
syndrome (an underlying cause of recurrent miscarriage), investigators also found no difference in the number of
prompted investigation of the effect of congenital pregnancies or fetal losses between women with and
activated protein C resistance on miscarriage Rai and those without thrombophilic partners.34 Sanson and
colleagues27 found a significantly higher prevalence of colleagues,35 found an increased incidence of pregnancy
such resistance in women with recurrent miscarriages loss in women with a deficiency of antithrombin, protein
(including at least one second-trimester loss) than in C, and protein S, with an odds ratio of 2·0 (1·2–3·3) for all
women with recurrent fetal loss in the first trimester or deficiencies combined. 35 Other thrombophilic defects such
in a parous control group (table 1). This link to fetal loss as dysfibrinogenaemia have also been linked to fetal loss.36
during the second trimester was supported by Grandone These data come from case-control or retrospective
and colleagues’ case-control study28 which showed a cohort studies. Prospective longitudinal studies are
significantly higher prevalence of factor V Leiden in needed to define the risk of pregnancy-related
recurrent miscarriage, particularly in the second complications in women with thrombophilic abnormalities.
trimester. Brenner and colleagues’ cohort study29 of Nonetheless, available data are consistent with the concept
women with recurrent fetal loss showed a 48% prevalence that maternal thrombophilia carries an increased risk of
of the factor V Leiden mutation; in women with factor V fetal loss due to placental vascular disorders.
Leiden, only 19% of 128 pregnancies ended in livebirths.
Other studies30–32 have shown no relation between Thrombophilia, intrauterine growth restriction,
recurrent miscarriage and factor V Leiden (table 2). and pre-eclampsia
These data from case-control and cohort studies suggest Since pre-eclampsia is associated with vascular injury,
that recurrent first-trimester miscarriage is not associated which in turn is associated with a disturbance in
with factor V Leiden, although second-trimester miscarriage coagulation, the congenital thrombophilias could have
may be. A possible explanation for this discrepancy is that a role in this disorder. Dekker and colleagues’ cohort
first-trimester miscarriages reflect a failure in implantation study37 of 101 patients with a history of pre-eclampsia
and second-trimester miscarriages reflect thrombotic event found that 15% of the women had underlying activated
in the placenta. The cohort study by Dizon-Townson and protein C resistance, although genotyping for factor V
colleagues30 showed a link with fetal genotype: 8·6% of Leiden was not done. These investigators also found that
1260 THE LANCET • Vol 353 • April 10, 1999
Type of thrombophilia Number of cases Odds ratio (95% CI)*
(pregnancies) All spontaneous fetal losses Miscarriages Stillbirths
Antithrombin deficiency 108 (250) 2·1 (1·2–3·6) 1·7 (1·0–2·8) 5·2 (1·5–18·1)
Protein C deficiency 162 (430) 1·4 (0·9–2·2) 1·4 (0·9–2·2) 2·3 (0·6–8·3)
Protein S deficiency 145 (378) 1·3 (0·8–2·1) 1·2 (0·7–1·9) 3·3 (1·0–11·3)
Factor V Leiden 141 (410) 1·0 (0·6–1·7) 0·9 (0·5–1·5) 2·0 (0·5–7·7)
Combined defects 15 (46) 2·0 (0·5–8·1) 0·8 (0·2–3·6) 14·3 (2·4–86·0)
Data are from refs 30–34.
Control group n=395 with 1019 pregnancies.
*Adjusted for number of pregnancies and centre (data from Preston and colleagues34).
Table 2: Odds ratios for fetal loss in women with thrombophilia compared with a control group by type of thrombophilia
25% of the women had a prevalence of protein S with pre-eclampsia, intrauterine growth restriction, or
deficiency, and that 18% of patients had a positive result both had factor V Leiden, compared with 10% of 465
to a methionine load test, which assesses the possibility healthy pregnant controls. In their case-control study of
of hyperhomocysteinaemia, Furthermore, 30% of women with pregnancy complications (pre-eclampsia,
patients had anticardiolipin antibodies.37 Several patients abruption, growth restriction, and stillbirth), Kupferminc
had combined disorders, but the only combination that and colleagues45 reported odds ratios of 3·7 (1·5–9·0), 3·1
recurred was protein S deficiency and a moderate (1·4–7·1), and 3·9 (1·1–14·6) in women with factor V
increase of anticardiolipin antibodies. De Vries and Leiden, homozygosity for the thermolabile variant of
colleagues38 assessed 62 women who had had placental MTHFR, and the prothrombin gene variant, respectively.
abruption, intrauterine death, or a small-for-gestational These data suggest that factor V Leiden and
age infant in the absence of antihypertensive disorders, homozygosity for the thermolabile variant of MTHFR
and found that 65%, 56%, and 85%, respectively, had predispose women to pre-eclampsia. Of interest is the
underlying thrombophilic disorders. Overall, 26% had fairly high rate (2–10%) of the factor V Leiden gene in
protein S deficiency, 24% of women had hyperhomo- the populations studied. Why such a genotype persists in
cysteinaemia, and 6% had protein C deficiency; no the population warrants further investigation. Analogies
women had a deficiency of antithrombin.38 Several case have been made with sickle-cell disease, in which the
reports have also described activated protein C resistance presence of sickle-cell trait affords protection against
in pregnancies complicated by HELLP syndrome malaria. Lindqvist and colleagues44 reported a fall in the
(haemolysis elevated liver enzymes and low platelets), a number of women with a blood loss at vaginal delivery of
variant of pre-eclampsia, severe pre-eclampsia, greater than 600 mL and the development of postpartum
intrauterine growth restriction, and fetal loss.29,39 Although anaemia in the women with factor V Leiden.
these studies point to a possible link between Activated protein C resistance can be acquired during
thrombophilia, pre-eclampsia, abruption, and intrauterine pregnancy, probably because of high concentrations of
growth restriction, interpretation is limited by the factor VIII and factor V.10 However, acquired resistance
observational design. However, some case-control studies does not account for all of the association of activated
have examined activated protein C resistance and factor protein C resistance with pre-eclampsia. The disturbance
V Leiden. of the coagulation system in pre-eclampsia means that
Dizon-Townson and colleagues40 case-control study vascular damage may result from the combination of
showed that 8·9% of women with severe pre-eclampsia an underlying thrombophilic disorder, the physiological
were heterozygous for the factor V Leiden mutation, changes in activated protein C resistance in pregnancy,
compared with 4·2% of controls. In another case-control and the pathological changes of pre-eclampsia.
study, Grandone and colleagues41 reported a prevalence
of 10·5% for factor V Leiden in women with pre- Screening for congenital thrombophilia
eclampsia versus 2·3% in controls, which gave an odds There is no evidence to support routine screening of
ratio of 4·9 (1·3–18·3). These researchers also assessed all pregnant women for congenital thrombophilia. The
underlying homozygosity for the thermolabile variant of natural history of many of the congenital thrombophilias
MTHFR: 29·8% of the pre-eclamptic patients had this in individuals is not known, nor is therapy for them.
variant, compared with 18·6% of controls, with an odds Moreover, the cost of screening is likely to be high. There
ratio of 1·8 (1·0–3·5). This finding suggests that is no doubt that screening is indicated for women with
hyperhomocysteinaemia may be a risk factor for pre- venous thromboembolism in pregnancy or who have a
eclampsia, presumably because of the toxic effects that personal or family history of this disorder. About 50% of
homocysteine has on the endothelium. If so, it may be such women will be found to have a thrombophilic
useful to measure folic acid, and vitamin B6 and B12 defect. Although the need for antenatal thrombo-
in women with adverse pregnancy outcome, since a prophylaxis may be uncertain for some disorders for
deficiency of these vitamins can lead to acquired example, symptomless factor V Leiden, the consensus is
hyperhomocysteinaemia, which is treatable with folic acid that women with a personal history of venous thrombo-
and vitamin B6 supplements.42 embolism and an underlying thrombophilic trait should
In a case-control study by Lindoff and colleagues43 receive thromboprophylaxis during pregnancy (see
22% of the 50 women with previous pre-eclampsia had below).
activated protein C resistance, compared with 10% of the There are no data to suggest that screening for
50 controls. There was no difference in concentrations of congenital thrombophilia in women with recurrent first-
protein C or antithrombin concentrations between the trimester fetal loss is justified, but patients with recurrent
groups and no association between intrauterine growth pregnancy loss, including a second-trimester miscarriage
restriction and activated protein C resistance. However, or a history of an intrauterine death, should be screened.
Lindqvist and colleagues 44 found that 18% of 122 women Screening is also justified for women with severe or
THE LANCET • Vol 353 • April 10, 1999 1261
recurrent intrauterine growth restriction or pre-eclampsia. Management of venous thromboembolism
However, in a patient with recurrent pregnancy loss The objective diagnosis of venous thromboembolism
and an underlying thrombophilic trait management is during pregnancy is crucial. The diagnosis has serious
uncertain. Some extrapolation can be made from the implications not only for management of the pregnancy,
benefits of aspirin plus heparin in women with but also for other features of the woman’s life, such
antiphospholipid antibody syndrome, but controlled trials as contraception and management of future pregnancies.
are needed to assess the effectiveness of such intervention All women with symptoms or signs compatible with
for women who screen positive. venous thromboembolism must have appropriate
investigation.
Anticoagulants and antithrombotic techniques Most events occur outside of hospital and carers of
Unfractionated and low-molecular-weight heparins do pregnant women in the community must be aware of this
not cross the placenta and, so, there is no risk of possibility and the underlying risk factors for venous
teratogenesis or fetal haemorrhage. These drugs are not thromboembolism. Real time or duplex ultrasonography
secreted in breastmilk and can be used during lactation. is the first-line diagnostic tool,54 particularly because
The disadvantages are osteoporosis, heparin-induced most cases of DVT are iliofemoral. If the initial
thrombocytopenia, and allergy.46,47 investigation is negative and there is continuing concern
Warfarin is not secreted in breastmilk in clinically about possible DVT, then the women should undergo
significant amounts and is safe to use during lactation. repeat ultrasonography. If pulmonary thromboembolism
However, this drug crosses the placenta, and warfarin is suspected then ventilation-perfusion lung scan should
embryopathy may occur in 4–5% of fetuses exposed to be done; in the first instance, perfusion scanning can
the drug between 6 and 9 weeks of gestation.48 Warfarin be used alone. Lung scans should be combined with
embryopathy can be prevented by substitution of heparin ultrasound venography. The radiation dose to the
for warfarin during the first trimester. Central-nervous- fetus from limited venography with abdominal
system abnormalities reported with warfarin exposure in shielding, ventilation-perfusion lung scan, and chest
the fetus may be due to spontaneous intracerebral radiography, even taken together, is small and deemed to
bleeding. Since the liver is immature and concentrations pose a negligible risk. If the diagnosis of pulmonary
of vitamin-K-dependent coagulation factors are low in thromboembolism remains uncertain after lung scan,
the fetus, maternal warfarin therapy maintained in the the results of ultrasonography are useful. If positive,
therapeutic range is associated with excessive anti- anticoagulant therapy should be started. Pulmonary
coagulation and potential bleeding in the fetus. Warfarin angiography is the gold standard for diagnosis of
should, particularly, be avoided beyond 36 weeks of pulmonary thromboembolism but is rarely necessary,
gestation,48,50 because of the excessive risk of haemorrhage although it should be considered in cases with a high
to both mother and fetus in the peripartum period. clinical probability of the disorder and inconclusive test
Dextran has been used for peripartum thrombo- reports. If there is a persistent clinical suspicion of venous
prophylaxis, particularly during caesarean section, and thromboembolism in the face of a negative or low-
carries a substantial risk of anaphylactic and anaphylactoid probability test result, treatment should be started and
reactions. Of greater concern in pregnancy is the risk of tests repeated within 7 days and therapy discontinued if
maternal anaphylactoid reactions associated with uterine tests remain negative.
hypertonus, profound fetal distress, and a high incidence Therapeutic doses of unfractionated or low-molecular-
of fetal death or profound neurological damage.51 Thus, weight heparin are used, 55 with thromboembolic deterrent
dextran should be avoided in pregnancy. stockings, and continued throughout the pregnancy.
Since thromboembolic-deterrent stockings are effective Twice daily subcutaneous administration is appropriate
in women who are not pregnant, they might be useful and the patient should be taught how to self-inject.
in pregnancy. These stockings stop overdistenstion The activated partial thromboplastin time is unreliable for
of veins and so prevent endothelial damage and exposure monitoring doses of unfractionated heparin in pregnancy
of subendothelial collagen.52 Other mechanical because of the pregnancy-associated increase in factor
techniques such as intermittent pneumatic compression VIII,51,55 so antifactor Xa concentrations may offer a
are of value during caesarean section and immediately better alternative way to measure heparin concentrations
postpartum. in pregnancy. The dose of low-molecular-weight heparin
Aspirin can prevent DVT,53 and its effectiveness in depends on maternal weight, and although monitoring
pregnancy, compared with heparin, remains to be is not required in the women who are not pregnant,
established. The effectiveness of aspirin is likely to be less measurement of peak antifactor Xa concentrations
than that of heparin and low-molecular-weight heparin. (target range 0·4–1·0 m/mL) may be of value until more
In women who are unable to take heparin or in whom the experience is gained of low-molecular-weight heparin
balance of risk is not deemed sufficient to merit heparin, in pregnancy.56 The dose of unfractionated and low-
aspirin may be useful. Low doses (60–75 mg daily) of molecular-weight heparin is reduced during delivery
aspirin are not associated with adverse pregnancy to prophylactic concentrations and the timing of
outcome. Hirudin is used in women who are not administration is adjusted to allow epidural or spinal
pregnant for treatment of thrombocytopenia induced by anaesthesia. Warfarin can be used postpartum,
heparin, but is also used for postoperative prophylaxis. particularly to avoid the risk of osteoporosis associated
Since hirudin crosses the placenta it should not be used with heparin, although many women prefer to stay on
in pregnancy; its use after delivery has not been assessed. low-molecular-weight heparin. Treatment should
The best option seems to be the established continue for at least 6 weeks and 3 months is often more
thromboprophylactic agents. appropriate.
1262 THE LANCET • Vol 353 • April 10, 1999
Thromboprophylaxis in pregnancy and the discussed above. In women with antithrombin deficiency,
puerperium the risk may be substantially greater and the dose of
All patients with a personal or family history of venous heparin needs to be adjusted throughout the pregnancy.
thromboembolism should be considered for antenatal Antithrombin preparations can be added at times of
prophylaxis and be screened for a thrombophilia. particularly high risk, such as delivery. Symptom-free
Thromboprophylaxis is also indicated when there are carriers of thrombophilia require special consideration. Since
additional risk factors, such as hyperemesis or surgery or the risk varies with the type of thrombophilia, all patients
when patients are obese or immobile, particularly if they with thrombophilia should be referred to a unit that
also have pre-eclampsia or concurrent medical conditions specialises in the management of thromophilia in pregnancy.
associated with thrombosis, such as nephrotic syndrome, During labour thromboprophylaxis should be
inflammatory bowel disease, or infection. If previous continued, but because of the risk of epidural haematoma
DVT is the only risk factor, antenatal anticoagulation is associated with heparin, the timing of the insertion (and
removal) of the epidural catheter, caesarean section, or
controversial, because of the potential hazards of heparin,
heparin dose may need to be adjusted to avoid peak
although use of low-molecular-weight heparin reduces
heparin concentration at the time of placement of the
the hazards. However, controlled trials are needed to
catheter.50 There have been postmarketing reports in the
guide management.
US Food and Drug Administration of low-molecular-
Anticoagulant therapy for thromboprophylaxis in
weight heparin and epidural haematoma. Most of these
pregnancy usually consists of unfractionated heparin
events have occurred in elderly women (median age 75
in a dose of 10 000 IU twice daily during the latter half of
years) undergoing orthopaedic surgery. Additional risk
pregnancy, or for low-molecular-weight heparin, 40 mg
factors have included concomitant use of non-steroidal
enoxaparin daily or 5000 IU dalteparin daily. There is no
anti-inflammatory agents or multiple puncture attempts
need to adjust the dose of low-molecular-weight heparin
at spinal or epidural. The true incidence of epidural
during pregnancy.45,57 Women with a bodyweight of
haematoma is impossible to determine because of lack of
less than 50 kg are likely to have satisfactory heparin
denominator data. In addition, the dose of enoxaparin
concentrations on low doses (20 mg enoxaparin, 2500 IU
given in Europe is 40 mg daily, compared with 30 mg
dalteparin),58 whereas obese women (>80 kg) may require twice daily in North America. However, caution is
higher doses. needed in giving epidural anaesthesia in patients on low-
In women with multiple thrombotic events during molecular-weight heparin. There must be vigilence for
previous pregnancies, antenatal prophylaxis should start signs of cord compression. Epidural anaesthesia must be
at least 4–6 weeks in advance of the gestation at which avoided in patients on therapeutic anticoagulation. In
the previous events occurred. If previous thrombosis was women on prophylactic doses of unfractionated and low-
not associated with pregnancy, then prophylaxis should molecular-weight heparin, epidural anaesthesia should be
start by about the mid point (20 weeks) of pregnancy or avoided around the time of peak-heparin concentrations.
sooner, particularly if additional risk factors are present. Thus placement of the epidural catheter must be delayed
Indeed, with the low incidence of side-effects with for 4 h with unfractionated heparin and longer with low-
low-molecular-weight heparin there is a case to start molecular-weight heparin the delay is arbitrary, since it is
prophylaxis from much earlier in pregnancy. Since the broadly extrapolated from the time of peak
risk of venous thromboembolism is likely to be high in concentrations and pharmacokinetics of the drugs. In my
patients with past thromboses, low-molecular-weight unit the delay is at least 6 h, but others suggest a delay of
or unfractionated heparin should be combined with 12 h. 60 Unfractionated and low-molecular-weight heparin
graduated elastic compression stockings. If heparin is can be restarted 2 h after catheter removal.
contraindicated, graduated elastic compression stockings Knowledge of thromboprophylaxis for women with no
and low-dose aspirin may be used. personal or family history of venous thromboembolism,
After delivery, thromboprophylaxis should be but with congenital thrombophilia and a history of fetal
continued for a minimum of 6 weeks, but in patients with loss or pre-eclampsia is limited. Aspirin is not beneficial
severe thrombocytic problems, for 3 months. Patients on in the prevention of pre-eclampsia. The combination of
heparin must be told of the risk of osteoporosis with low-dose aspirin and heparin is effective in preventing
heparin. If the patient wants to change to warfarin it can recurrent fetal loss in women with anticardiolipin
be started 48 h after delivery with the heparin continued antibodies,4 and could be considered for women with
until the international normalised ratio is between 2 congenital thrombophilia and fetal loss, pre-eclampsia, or
and 2·5. Unfractionated heparin is prescribed in a dose of both.
7500–10 000 IU twice daily, and low-molecular-weight- Caesarean section, particularly emergency caesarean
heparin such as enoxaparin or dalteparin in doses of section, carries substantial increased risk of
40 mg or 5000 IU daily, respectively. Gibson and thromboembolic disease and guidelines have been drawn
colleagues59 reported that low-molecular-weight heparin up for management according to risk.61 Patients at low
provided better antifactor Xa concentrations than did risk are those who undergo elective caesarean section
unfractionated heparin in conventional doses after with an uncomplicated pregnancy and no additional risk
caesarean section.59 factors. Women at moderate risk are those who undergo
Many patients with underlying congenital or acquired caesarean section and have another risk factor such as age
thrombophilia will require antenatal prophylaxis, the older than 35 years or obesity, or if the caesarean section
timing of which will depend on the patient’s history and is an emergency procedure. Women at high risk are those
thrombophilic disorder.14 In patients with symptomatic with multiple risk factors or severe disorders such as
protein C deficiency, protein S deficiency, and factor V thrombophilia or paralysis of the lower limbs. These
Leiden, standard treatment is unfractionated or low- women should receive unfractionated or low-molecular-
molecular-weight heparin antenatally in the doses weight heparin thromboprophylactically; duration of
THE LANCET • Vol 353 • April 10, 1999 1263
therapy depends on the magnitude of the risk. In view of gene contributing to venous thrombosis in pregnancy. Br J Obstet
Gynaecol 1998; 105: 923–25.
the increase in death after vaginal delivery, guidelines
19 Den Heijer M, Koster T, Blom HJ, et al. Hyperhomocysteinemia as a
should also be developed for the management of women risk factor for deep vein thrombosis. N Engl J Med 1998; 334: 759–62.
with risk factors after vaginal delivery. 20 Conard J, Horellou MH, van Dreden P, Le Compte T, Samama M.
Thrombosis in pregnancy and congenital deficiencies in AT III,
protein C or protein S: study of 78 women. Thromb Haemost 1990;
Conclusion 63: 319–20.
Thrombosis in pregnancy and underlying thrombophilia 21 de Stefano V, Leone G, Masterangela S, et al. Thrombosis during
have serious implications for mother and fetus. Evidence pregnancy and surgery in patients with congenital deficiency of anti-
thrombin III, protein C–protein S. Thromb Haemost 1994; 71:
is accumulating to link congenital thrombophilia to fetal 799–800.
loss, intrauterine growth restriction, pre-eclampsia and 22 Hough RE, Makris N, Preston FR. Pregnancy in women with
maternal venous thromboembolism. However, the thrombophilia: incidence of thrombosis and pregnancy outcome.
Br J Haematol 1996; 93 (suppl 2): 136 (abstr).
knowledge is based mostly on case-control and cohort
23 Pabinger I and the Study Group on Natural Inhibitors. Thrombotic
studies, so the natural history of these conditions in risk in hereditary anti-thrombin III, protein C or protein S deficiency.
symptom-free individuals is uncertain and large-scale, Arter Thromb Vasc Biol 1996; 16: 742–48.
prospective, longitudinal studies are needed. Only when 24 Hallak M, Senderowica J, Cassel A, et al. Activated protein C
resistance (factor V Leiden) associated ith thrombosis in pregnancy.
the implications of thrombophilic defects are known, Am J Obstet Gynecol 1997; 176: 889–93.
can appropriate intervention be assessed, ideally in large 25 Bokarewa MI, Bremme K, Blomback M. Arg 506-Gln mutation in
randomised trials. In the meantime, women with a factor V and risk of thrombosis during pregnancy. Br J Haematol 1996;
personal or family history of venous thromboembolism, 92: 473–78.
26 de Stefano V, Leone G, Masterangelo S, et al. Thrombotic risk during
second-trimester fetal loss, intrauterine death, and severe pregnancy and puerperium in women with APC resistance: effective
or recurrent intrauterine growth restriction and pre- subcutaneous heparin prophylaxis in a pregnant patient. Thromb
eclampsia, should be screened for thrombophilia and Haemost 1995; 74: 793–94.
consideration given to thromboprophylaxis. 27 Rai RS, Regan L, Hadley E, Dave M, Cohen H. Second trimester
pregnancy loss is associated with activated Protein C resistance. Br J
Haematol 1996; 92: 489–90.
References 28 Grandone E, Margaglione M, Colaizzo D, et al. Factor V Leiden is
associated with repeated and recurrent explained fetal losses. Thromb
1 Department of Health, Welsh Office, Scottish Home and Health Haemostas 1997; 77: 822–24.
Department and Department of Health and Social Services Northern
29 Brenner B, Mandel H, Lanir N, Younis J, Rothbart H. Activated
Ireland. Confidential Enquiries into Maternal Deaths in the United
protein C resistance can be associated with recurrent fetal loss. Br J
Kingdom 1994–97. London: TSO, 1998.
Haematol 1997; 97: 551–54.
2 Greer IA, de Swiet M. Thrombosis prophylaxis in obstetrics and
30 Dizon-Townson DS, Meline L, Nelson LM, Barner M, Ward K. Fetal
gynaecology. Br J Ostet Gynaecol 1993; 100: 37–40.
carriers of the factor V Leiden mutation are prone to miscarriage and
3 Vincent T, Rai R, Regan L, Cohen H. Increased thrombin generation placental infarction. Am J Obstet Gynecol 1997; 177: 402–05.
in women with recurrent miscarriage. Lancet 1998; 353: 116.
31 Dizon-Townson DS, Kinney S, Branch DW, Ward K. The factor V
4 Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of Leiden mutation is not a common cause of recurrent miscarriage.
aspirin and aspirin plus heparin in pregnant women with recurrent J Reprod Immunol 1997; 34: 217–23.
miscarriage associated with phospholipid antibodies (or
32 Belasch J, Riverter JC, Fabregues F, et al. First trimester repeated
antiphospholipid antibodies). BMJ 1997; 314: 253–57.
abortion is not associated wtih activated protein C resistance. Hum
5 Greaves M. Antiphospholipid antibodies and thrombosis. Lancet Reprod 1997; 12: 1094–97.
(in press). 33 Rai RS, Regan L, Chitolie A, Donald JG, Cohen H. Placental
6 Macklon NS, Greer IA. Venous thromboembolic disease in obstetrics thrombosis in second trimester miscarriage in association with activated
and gynaecology: the Scottish experience. Scot Med J 1996; 41: 83–86. Protein C resistance. Br J Obstet Gynaecol 1996; 103: 842–44.
7 Rutherford S, Montoro M, McGhee W, Strong T. Thromboembolic 34 Preston FE, Rosendaal FR, Walker ID, et al. Increased fetal loss in
disease associated with pregnancy: an 11 year review. Am Obstet women with heritable thrombophilia. Lancet 1996; 348: 913–16.
Gynecol 1991; 164 (suppl): 286. 35 Sanson BJ, Friederich PW, Simioni P, et al. The risk of abortion and
8 McColl M, Ramsay JE, Tait RC, et al. Risk factors for pregnancy stillbirth in antithrombin, Protein S and Protein S deficient women.
associated venous thromboembolism. Thromb Haemost 1997; 78: Thromb Haemost 1996; 75: 387–88.
1183–88. 36 Havercate F, Samama M. Familial dysfibrinogenaemia and
9 Lindhagen A, Bergqvist A, Bergqvist D, Hallbook T. Late venous thrombophilia—report on a study of the SSC sub-committee on
function in the leg after deep venous thrombosis occurring in relation fibrinogen. Thromb Haemostas 1995; 73: 151–61.
to pregnancy. Br J Obstet Gynaecol 1986; 93: 348–52. 37 Dekker GA, de Vries JIP, Doelitzsch PM, et al. Underlying disorders
10 Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. associated with severe early onset of pre-eclampsia. Am J Obstet
Activated protein C sensitivity, protein C, protein S and coagulation Gynecol 1996; 173: 104–45.
in normal pregnancy. Thromb Haemost 1998; 79: 1166–70. 38 De Vries JIP, Dekker J, Huijgens PC, Jakobs C, Blomberg BME, van
11 Greer IA. Haemostasis and thrombosis in pregnancy. In: Bloom AL, Geijn HP. Hyperhomocysteinaemia and Protein S deficiency in
Forbes CD, Thomas DP, Tuddenham EGD, eds. Haemostasis and complicated pregnancies. Br J Obstet Gynaecol 1997; 104: 1248–54.
thrombosis. Edinburgh: Churchill Livingstone, 1994: 987–1015. 39 Rotmensch S, Liberati M, Mittlemann M, Ben-Rafael Z. Activated
12 Macklon NS, Greer IA, Bowman AW. An ultrasound study of protein C resistance and adverse pregnancy outcome. Am J Obstet
gestational and postural changes in the deep venous system of the leg Gynecol 1997; 177: 170–73.
in pregnancy. Br J Obstet Gynaecol 1997; 104: 191–97. 40 Dizon-Townson DS, Nelson LM, Easton K, Ward K. The factor V
13 Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet Leiden mutation may predispose women to severe pre-eclampsia.
1999; 353: 1167–73. Am J Obstet Gynaecol 1996; 175: 902–05.
14 Walker ID. Congenital thrombophilia. In: Greer IA, ed. Bailliere’s 41 Grandone E, Margagilione M, Colazzo D, et al. Factor V Leiden.
clinical obstetrics and gynaecology—thromboembolic disease in C>TMTHFR polymorphism and genetic susceptibility to pre-
obstetrics and gynaecology. London: Bailliere Tindall, 1997: 431–45. eclampsia. Thromb Haemostas 1997; 77: 1052–54.
15 Zoller B, Holm J, Dahlback B. Resistance to activated protein C due 42 Leeda M, Riyazi N, de Vries JIP, Jakobs C, van Geijn HP,
to factor V gene mutation: the most common inherited risk factor of Dekker GA. Effects of folic acid and vitamin B6 supplementation on
thrombosis. Trends Cardiovasc Med 1996; 6: 45. women with hyperhomocysteinemia and a history of pre-eclampsia or
16 Bokarewa MI, Wramsby N, Bremme K, Blomback M. Variability of fetal growth restriction. Am J Obstet Gynecol 1998; 179: 136–39.
the response to activated protein C during normal pregnancy. Blood 43 Lindoff C, Ingemarsson I, Martinsson G, Segelmark M, Thysell H,
Coag Fibrinol 1997; 8: 239. Astedt B. Pre-eclampsia is associated with a reduced response to
17 Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common activated protein C. Am J Obstet Gynecol 1997; 176: 457–60.
genetic variation in the three untranslated region of the prothrombin 44 Lindqvist PG, Svensson PJ, Dahlback B, Masal K. Factor V Q506
gene is associated with elevated plasma prothrombin levels and an mutation (activated protein C resistance) associated with reduced
increase in venous thrombosis. Blood 1996; 88: 36–38. intrapartum blood loss—a possible evolutionary selection mechanism.
18 McColl MD, Walker ID, Greer IA. A mutation in the prothrombin Thromb Haemost 1998; 79: 69–73.
1264 THE LANCET • Vol 353 • April 10, 1999
45 Kupferminc MJ, Amiram E, Steinman N, et al. Increased frequency of 53 Thrombombolic risk factors (THRIFT) Consensus Group. Risk of
genetic thrombophilia in women with complications of pregnancy. and prophylaxis for venous thromboembolism in hospital patients.
N Engl J Med 1999; 1: 9–13. BMJ 1992; 305: 567–74.
46 Macklon NS, Greer IA, Reid AW, Walker ID. Thrombocytopenia 54 Macklon NS. Diagnosis of deep venous thrombosis and pulmonary
antithrombin deficiency and extensive thromboembolism in embolism. In: Greer IA, eds. Bailliere’s clinical obstetrics and
pregnancy—treatment with low molecular weight heparin. Blood gynaecology—thromboembolic disease in obstetrics and gynaecology.
Coag Fibrinol 1995; 6: 672–75. London: Bailliere Tindall, 1997: 463º77.
47 Nelson-Piercy C. Hazards of heparin: allergy, heparin-induced 55 Lowe GDO. Treatment of venous thromboembolism. In: Greer IA,
thrombocytopenia and osteoporosis. In: Greer IA, ed. Bailliere’s ed. Bailliere’s clinical obstetrics and gynaecology—thromboembolic
clinical obstetrics and gynaecology—thromboembolic disease in disease in obstetrics and gynaecology. London: Bailliere Tindall, 1997:
obstetrics and gynaecology. London: Bailliere Tindall, 1997: 489–509. 511–21.
48 Bates SM, Ginsberg JS. Anticoagulants in pregnancy: fetal defects. In: 56 Thomson AJ, Walker ID, Greer IA. Low molecular weight heparin for
Greer IA, ed. Bailliere’s clinical obstetrics and gynaecology— immediate management of thromboembolic disease in pregnancy.
thromboembolic disease in obstetrics and gynaecology. London: Lancet 1998; 352: 1904.
Bailliere Tindall, 1997: 479–88. 57 Brennand JE, Walker ID, Greer IA. Anti-activated factor X profiles in
49 Warkentin TE, Levine MN, Hisch J, et al. Heparin induced pregnant women receiving antenatal thromboprophylaxis with
thrombocytopenia in patients treated wtih low molecular weight Enoxaparin. Acta Haematol 1999; 101: 53–55.
heparin or unfractionated heparin. N Engl J Med 1995; 332: 1330–35. 58 Blomback M, Bremme K, Hellgren M, Lindberg H. A
50 Letsky E. Peripartum prophylaxis of thromboembolism. In: Greer IA, pharmacokinetic study of dalteparin (Fragmin) during late pregnancy.
ed. Bailliere’s clinical obstetrics and gynaecology—thromboembolic Blood Coag Fibrinol 1998; 9: 343–50.
disease in obstetrics and gynaecology. London: Bailliere Tindall, 1997: 59 Gibson JL, Ekevall K, Walker ID, Greer IA. Puerperal
23–43. thromboprophylaxis: comparison of the anti-Xa activity of enoxaparin
51 Greer IA. Epidemiology, risk factors, and prophylaxis of venous and unfractionated heparin. Br J Obstet Gynaecol 1998; 105: 795–97.
thromboembolism in obstetrics and gynaecology. In: Greer IA, ed. 60 Horlocker TT, Heit JA. Low molecular weight heparin:
Bailliere’s clinical obstetrics and gynaecology—thromboembolic biochemistry, pharmacology, perioperative prophylaxis regimens and
disease in obstetrics and gynaecology. London: Bailliere Tindall, 1997: guidelines for regional anesthetic management. Anesth Analg 1997; 85:
403–30. 874–85.
52 Macklon NS, Greer IA. Technical note: compression stockings and 61 Report of the RCOG Working Party on Prophylaxis Against
posture—a comparative study of their effects on the proximal deep Thromboemolism in Gynaecology and Obstetrics. London: Royal
veins in the leg at rest. Br J Radiol 1995; 68: 515–18. College of Obstetricians and Gynaecologists, 1995.
Essay
Helen
Peter Reagan
She pointed to her left cheek and said, “You can kiss me oxygen, but completely astute, with a delightful twinkle; I
now. Right here”. Taken by surprise, and extremely liked her immediately. She and her family carefully
moved, I kissed her lightly and sat back. She smiled and detailed her history of breast cancer, her years since her
looked up brightly. Afterwards she asked me for some first mastectomy, the recurrence on the other side, the
brandy. Beth had diluted it fifty-fifty with a soft drink. metastases, and the downhill course. The second tumour
She wasn’t used to drinking much alcohol, and she had developed when she was 82; she had refused all
choked on it just a little as it went down. Beth offered her treatment for it after the lumpectomy. Her husband had
a mint, but she waved it away and said she was sleepy. suffered a lingering death a decade earlier; she was opting
Douglas held her right hand and arm, as I held her left. for life while it was good and then a clean exit.
Beth massaged her feet. Her breathing was slow, even, I discussed the requirements of the law with her. When
and relaxed. About a minute later I asked her how she I got to the part about a written request, she handed me a
was doing and she mumbled, “Tired”. We took two of notarised statement signed by Douglas and a neighbour. I
the pillows from behind her head so she could sit back looked into her eyes and shivered as I contemplated the
more easily, and Helen fell into a deep sleep. enormity of what I was facing. I took a deep breath and
The three of us sat around her bed talking quietly we started discussing her attempts to get help with dying.
about the emotional struggle we’d each been through. 3 She had asked her original physician, but he was about to
weeks earlier, I had received a phone call from a retired relocate his practice and didn’t want to be involved. He
colleague about a patient who wanted help ending her life had recommended hospice care and had referred her to a
under the new Oregon Death With Dignity Law (see second primary physician who, he thought, might have
Lancet 1994; 344: 1493–94). Her previous physicians had been open to aid in dying. The second physician had seen
not been prepared to offer a life-ending prescription. her twice, ordered a chest radiograph, and also
Could I see her soon? encouraged hospice care. He agreed that she had a short
5 days later she was sitting in a wheelchair in an life-expectancy but recorded in her notes that she was
examination room with her son Douglas and her probably depressed. Helen and her family felt he was not
daughter Beth. She was elderly, frail, and hooked to ready to provide assistance and was buying time. She
said, “He made me wait 24 days, and he never intended
Lancet 1999; 353: 1265–67 to help me. I think he still expected me to come back to
2406 NE 19th Avenue, Portland, OR 97212, USA (P Reagan MD) him. He should have told me at the beginning”.
THE LANCET • Vol 353 • April 10, 1999 1265
You might also like
- Review Article: The Role of Thrombophilia in PregnancyDocument14 pagesReview Article: The Role of Thrombophilia in PregnancyTina GrosuNo ratings yet
- Management of Venous Thrombosis in PregnancyDocument7 pagesManagement of Venous Thrombosis in PregnancyAita AladianseNo ratings yet
- Embarazo, Anticoagulacion y Bloqueos NeuroaxialesDocument22 pagesEmbarazo, Anticoagulacion y Bloqueos Neuroaxialesiveca64No ratings yet
- Management of Venous Thromboembolism in PregnancyDocument21 pagesManagement of Venous Thromboembolism in PregnancyShigma Putra MahaleyNo ratings yet
- Background: Placental Abruption Seen After DeliveryDocument8 pagesBackground: Placental Abruption Seen After DeliverymoigomezNo ratings yet
- Background: Placental Abruption Seen After DeliveryDocument8 pagesBackground: Placental Abruption Seen After DeliverymoigomezNo ratings yet
- 2020 - Maternal Inherited Thrombophilia and Pregnancy OutcomesDocument4 pages2020 - Maternal Inherited Thrombophilia and Pregnancy OutcomesIrina SerpiNo ratings yet
- Postpartum Maternal Mortality and Pulmonary Embolism: A Case ReportDocument3 pagesPostpartum Maternal Mortality and Pulmonary Embolism: A Case ReportSabrina JonesNo ratings yet
- Pregnancy and CirrhosisDocument11 pagesPregnancy and CirrhosisSitha MahendrataNo ratings yet
- Clinical Considerations in Recurrent Miscarriage: Journal ofDocument5 pagesClinical Considerations in Recurrent Miscarriage: Journal ofElena VisterniceanNo ratings yet
- Anticoagulation in Pregnancy ComplicationsDocument7 pagesAnticoagulation in Pregnancy ComplicationsAlena NovikovaNo ratings yet
- Maedica 11 55 PDFDocument6 pagesMaedica 11 55 PDFM Iqbal EffendiNo ratings yet
- Thrombophilia-Associated Pregnancy Wastage: Modern TrendsDocument10 pagesThrombophilia-Associated Pregnancy Wastage: Modern TrendspogimudaNo ratings yet
- Thrombosis in Pregnancy - Updates in Diagnosis and Management 2013 0Document5 pagesThrombosis in Pregnancy - Updates in Diagnosis and Management 2013 0Aita AladianseNo ratings yet
- Thromboembolism in Pregnancy: Victor A. Rosenberg, MD, Charles J. Lockwood, MDDocument20 pagesThromboembolism in Pregnancy: Victor A. Rosenberg, MD, Charles J. Lockwood, MD10019kNo ratings yet
- Antepartum Haemorrhage: ReviewDocument6 pagesAntepartum Haemorrhage: ReviewBodat BodatsNo ratings yet
- Embolismo Pulonar y Liquido Amniotico Sept 2022Document22 pagesEmbolismo Pulonar y Liquido Amniotico Sept 2022rafael martinezNo ratings yet
- Obstetrics V13 Obstetric Emergencies Chapter Pulmonary Embolism During Pregnancy 1632545155Document8 pagesObstetrics V13 Obstetric Emergencies Chapter Pulmonary Embolism During Pregnancy 1632545155Miriam Sanchez RamosNo ratings yet
- Stroke in Pregnancy and The Puerperium: S D Treadwell, B Thanvi, T G RobinsonDocument8 pagesStroke in Pregnancy and The Puerperium: S D Treadwell, B Thanvi, T G RobinsonNabillah MukhlisNo ratings yet
- Prof. Dr. Farhana Dewan, Dr. Mariha Alam Chowdhury, Dr. Khairun NessaDocument6 pagesProf. Dr. Farhana Dewan, Dr. Mariha Alam Chowdhury, Dr. Khairun Nessasumaiya zaforNo ratings yet
- Abruptio Placentae DIC 2009Document7 pagesAbruptio Placentae DIC 2009radistryaNo ratings yet
- TBL4, VTE in Pregnancy and Puerperium. RashidDocument5 pagesTBL4, VTE in Pregnancy and Puerperium. Rashidmedical chroniclesNo ratings yet
- Diagnosis and Management of Deep Vein Thrombosis in PregnancyDocument10 pagesDiagnosis and Management of Deep Vein Thrombosis in PregnancyAita AladianseNo ratings yet
- Amokrane2016 PDFDocument5 pagesAmokrane2016 PDFRaditya TaslimNo ratings yet
- DownloadDocument19 pagesDownloadRufino Guarniz Capristan100% (1)
- Challenges of Anticoagulkation Therapy in PregnancyDocument13 pagesChallenges of Anticoagulkation Therapy in PregnancyChicinaș AlexandraNo ratings yet
- Trombocitopenia GestazDocument6 pagesTrombocitopenia GestazSilvia Di GiovanniNo ratings yet
- Inherited and Acquired Thrombophilia - Pregnancy Outcome and TreatmentDocument22 pagesInherited and Acquired Thrombophilia - Pregnancy Outcome and TreatmentLuiza RadulescuNo ratings yet
- GTG37 A Reducing Risk ThrombosisDocument35 pagesGTG37 A Reducing Risk ThrombosisNacho AdiegoNo ratings yet
- Trombocitopenia Embarazo 2016Document11 pagesTrombocitopenia Embarazo 2016piloricoNo ratings yet
- Article 262063-PrintDocument15 pagesArticle 262063-PrintAhsan AuliyaNo ratings yet
- Antepartum and Postpartum Hemorrhage: Fitsum AshebirDocument64 pagesAntepartum and Postpartum Hemorrhage: Fitsum Ashebirzuzuyasi65No ratings yet
- Incomplete AbortionDocument18 pagesIncomplete AbortionAra DirganNo ratings yet
- Threatened AbortionDocument4 pagesThreatened AbortionKate VillalonNo ratings yet
- Abruptio PlacentaeDocument18 pagesAbruptio PlacentaeRitamariaNo ratings yet
- Recurrent Miscarriage EpidemiologymmmDocument7 pagesRecurrent Miscarriage EpidemiologymmmrhinaNo ratings yet
- Syphilis Ans PregnancyDocument7 pagesSyphilis Ans PregnancyviviocaNo ratings yet
- Management of Placenta Previa During PregnancyDocument5 pagesManagement of Placenta Previa During PregnancyUlayya Ghina NabillaNo ratings yet
- Midwifery Triage of First Trimester Bleeding: Susan A. Krause,, and Barbara W. GravesDocument12 pagesMidwifery Triage of First Trimester Bleeding: Susan A. Krause,, and Barbara W. GravesRival Risvaldi RusliNo ratings yet
- PIIS0002937823005136Document14 pagesPIIS0002937823005136Familia Valdivia VilcaNo ratings yet
- Ngeh 2006Document5 pagesNgeh 2006ayu fitriaNo ratings yet
- Clinical Review - FullDocument4 pagesClinical Review - FullPutriNo ratings yet
- TOG Thromboprophylaxis in Gynaecology A Review of Current EvidenceDocument13 pagesTOG Thromboprophylaxis in Gynaecology A Review of Current EvidenceMarNo ratings yet
- Antepartum Hemorrhage: Learning Objectives by The End of This Chapter, The Participant WillDocument12 pagesAntepartum Hemorrhage: Learning Objectives by The End of This Chapter, The Participant WillMelissa Aina Mohd YusofNo ratings yet
- TEP EmbarazoDocument14 pagesTEP EmbarazoOliver AguirreNo ratings yet
- Table 2: Maternal Indications For Termination of The Desired PregnancyDocument5 pagesTable 2: Maternal Indications For Termination of The Desired PregnancyMenaKamelNo ratings yet
- Pathologic OBDocument3 pagesPathologic OBAngielyn P. VicenteNo ratings yet
- Preterm Premature (Prelabor) Rupture of MembranesDocument26 pagesPreterm Premature (Prelabor) Rupture of MembranesAlejandro CadizNo ratings yet
- Stillbirth Eisit A Preventable Public Health Problem in The 21st Century?Document6 pagesStillbirth Eisit A Preventable Public Health Problem in The 21st Century?Yulinar Budi AdhiNo ratings yet
- Stillbirth Eisit A Preventable Public Health Problem in The 21st Century?Document6 pagesStillbirth Eisit A Preventable Public Health Problem in The 21st Century?Yulinar Budi AdhiNo ratings yet
- Background: Abruptio PlacentaeDocument6 pagesBackground: Abruptio PlacentaeEj ZGNo ratings yet
- Thrombocytopenia in PregnancyDocument13 pagesThrombocytopenia in PregnancyJhuli Elizabeth CNo ratings yet
- Inggris SoalDocument23 pagesInggris SoalZakia AmeliaNo ratings yet
- Pregnancy and Cirrhosis: ReviewDocument11 pagesPregnancy and Cirrhosis: ReviewBahrunNo ratings yet
- Thrombocytopenia in PregnancyDocument12 pagesThrombocytopenia in PregnancyzoyaNo ratings yet
- Thrombocytopenia in PregnancyDocument18 pagesThrombocytopenia in PregnancyDavid Eka PrasetyaNo ratings yet
- Contemporary Diagnosis and Management of Preterm Premature Rupture of MembranesDocument12 pagesContemporary Diagnosis and Management of Preterm Premature Rupture of Membranesriena456No ratings yet
- Placenta PreviaDocument8 pagesPlacenta PreviaBj DuquesaNo ratings yet
- Hysterectomy A-Z: Why, When, How and What afterFrom EverandHysterectomy A-Z: Why, When, How and What afterRating: 4 out of 5 stars4/5 (2)
- Molecular Cytogenetic Analysis of A DuplDocument7 pagesMolecular Cytogenetic Analysis of A DuplgeorgianaNo ratings yet
- Poursadegh Zonouzi Et Al - 2013 - The Association Between Thrombophilic Gene Mutations and Recurrent PregnancyDocument8 pagesPoursadegh Zonouzi Et Al - 2013 - The Association Between Thrombophilic Gene Mutations and Recurrent PregnancygeorgianaNo ratings yet
- Significance of 17 Variant Chromosome in Three FamiliesDocument7 pagesSignificance of 17 Variant Chromosome in Three FamiliesgeorgianaNo ratings yet
- Forensic Dna Statistic Evett I Weir PDFDocument306 pagesForensic Dna Statistic Evett I Weir PDFMirko StambolićNo ratings yet
- Interpreting DNA Evidence Statistical Genetics ForDocument3 pagesInterpreting DNA Evidence Statistical Genetics ForgeorgianaNo ratings yet
- Forensic Dna Statistic Evett I Weir PDFDocument306 pagesForensic Dna Statistic Evett I Weir PDFMirko StambolićNo ratings yet
- Bijnens Et Al 2019Document9 pagesBijnens Et Al 2019georgianaNo ratings yet
- A Computerized Model For Prediction of RDocument1 pageA Computerized Model For Prediction of RgeorgianaNo ratings yet
- Significance of 17 Variant Chromosome in Three FamiliesDocument7 pagesSignificance of 17 Variant Chromosome in Three FamiliesgeorgianaNo ratings yet
- Bijnens Et Al 2019Document9 pagesBijnens Et Al 2019georgianaNo ratings yet
- Early Follicular Phase LH Concentration PDFDocument7 pagesEarly Follicular Phase LH Concentration PDFgeorgianaNo ratings yet
- Isolation and Characterization of DNADocument5 pagesIsolation and Characterization of DNAgeorgiana0% (1)
- Intech NGS Imp PDFDocument59 pagesIntech NGS Imp PDF@lsreshtyNo ratings yet
- Allele Specific Amplification by Tetra-Primer PCRDocument1 pageAllele Specific Amplification by Tetra-Primer PCRgeorgianaNo ratings yet
- Isolation and Characterization of DNADocument5 pagesIsolation and Characterization of DNAgeorgiana0% (1)
- Isolation of DNADocument19 pagesIsolation of DNAgeorgianaNo ratings yet
- Isolation of DNADocument19 pagesIsolation of DNAgeorgianaNo ratings yet
- Obat Induksi HIPERURISEMIA A PDFDocument10 pagesObat Induksi HIPERURISEMIA A PDFshelfinararaNo ratings yet
- JURISDocument15 pagesJURISSharmaine Juco100% (1)
- Information MSQ KROK 2 Medicine 2007 2021 NEUROLOGYDocument92 pagesInformation MSQ KROK 2 Medicine 2007 2021 NEUROLOGYReshma Shaji PnsNo ratings yet
- Essential Oils and Supplements For Strokes: StrokeDocument4 pagesEssential Oils and Supplements For Strokes: StrokeEmese Földesi100% (1)
- Dataset On Child Vaccination in Brazil From 1996 To 2021: Data DescriptorDocument9 pagesDataset On Child Vaccination in Brazil From 1996 To 2021: Data DescriptorlaireneNo ratings yet
- Antimicrobial Dressings Made Easy PDFDocument6 pagesAntimicrobial Dressings Made Easy PDFNinaNo ratings yet
- DR Eric and Sabrina Zielinski Non Toxic Cleaners Diy RecipeDocument159 pagesDR Eric and Sabrina Zielinski Non Toxic Cleaners Diy RecipefizzNo ratings yet
- Intermittent Fasting Vs Daily Calorie Restriction For Type 2 Diabetes Prevention: A Review of Human FindingsDocument10 pagesIntermittent Fasting Vs Daily Calorie Restriction For Type 2 Diabetes Prevention: A Review of Human FindingsAmry Irsyada YusufNo ratings yet
- Ocular Fundus DrawingDocument33 pagesOcular Fundus DrawingguhanNo ratings yet
- PDF History of Medical Technology Profession CompressDocument5 pagesPDF History of Medical Technology Profession CompressJose KruzNo ratings yet
- Tuansi-Pa ManuscriptDocument11 pagesTuansi-Pa Manuscriptallkhusairy6tuansiNo ratings yet
- Milady Standard Esthetics Advanced: Advanced Skin Care MassageDocument72 pagesMilady Standard Esthetics Advanced: Advanced Skin Care Massagechristina magallanesNo ratings yet
- Naomi Williams FindingsDocument58 pagesNaomi Williams Findingsscott.faulkner.taylorNo ratings yet
- Sample Essay On The Negative Effects of TelevisionDocument2 pagesSample Essay On The Negative Effects of TelevisionkagurasNo ratings yet
- Emergency Procedures and Primary Care in Physical Therapy: A Practice ManualDocument32 pagesEmergency Procedures and Primary Care in Physical Therapy: A Practice ManualBushra MehwishNo ratings yet
- Ensuring Patient Comfort and Clinical Accuracy During Impression Taking 3Document11 pagesEnsuring Patient Comfort and Clinical Accuracy During Impression Taking 3Raul HernandezNo ratings yet
- Child Abuse and Neglect-Dr - Anisha NandaDocument49 pagesChild Abuse and Neglect-Dr - Anisha NandaHaripriya SukumarNo ratings yet
- Family Abuse and Neglect HandoutDocument9 pagesFamily Abuse and Neglect Handoutapi-498295251No ratings yet
- Dermawound Original Venous Stasis Wound CareDocument8 pagesDermawound Original Venous Stasis Wound CareNew Medical Solutions - Your Source for Dermawound, BurnBGone and more...No ratings yet
- Csir Life Sciences Fresh Instant NotesDocument4 pagesCsir Life Sciences Fresh Instant NotesAlps Ana33% (3)
- Literature Review On Oxygen TherapyDocument6 pagesLiterature Review On Oxygen Therapyjzneaqwgf100% (1)
- Research Material PDFDocument125 pagesResearch Material PDFNikita NelsonNo ratings yet
- Stroke AHA GuidelinesDocument104 pagesStroke AHA GuidelinesCristina ZeamaNo ratings yet
- 2000 Microbiology MCQs With KeyDocument100 pages2000 Microbiology MCQs With KeyAbdurrahman ZahidNo ratings yet
- Ocupol D Drops and Ointment PDFDocument3 pagesOcupol D Drops and Ointment PDFAnushka ShresthaNo ratings yet
- Region: Arum TriphyllumDocument3 pagesRegion: Arum TriphyllumKamalNo ratings yet
- CA Scal Provider DirectoryDocument180 pagesCA Scal Provider DirectorykevindamasoNo ratings yet
- Task 2 Engineering English (Redho Triwinoko, METO 5B)Document3 pagesTask 2 Engineering English (Redho Triwinoko, METO 5B)Redho TriwinokoNo ratings yet
- HEALTH-TEACHING-PLAN sUGATON EVALDocument9 pagesHEALTH-TEACHING-PLAN sUGATON EVALPrincess Faniega SugatonNo ratings yet
- Congenital Heart DiseaseDocument43 pagesCongenital Heart DiseaseSanjanaNo ratings yet