Professional Documents
Culture Documents
DPP-H Bonding
Uploaded by
Shivam Roy0 ratings0% found this document useful (0 votes)
7 views1 pageOriginal Title
DPP-H+bonding
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views1 pageDPP-H Bonding
Uploaded by
Shivam RoyCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
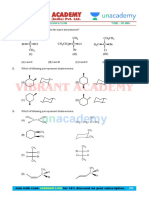
DPP
Q.1 Explain the structure of Boric acid in solid state.
Q.2 Boiling point of o-Nitrophenol is less than meta and para nitrophenol. Why?
Q.3 Maleic acid is more acidic than fumaric acid. Why?
Q.4 H – F is only liquid among halogen acid. Why?
Q.5 Ammonia is more easily liquefied than HCl, explain.
Q.6 Why ice floats on water?
Q.7 Water shows maximum density at 4°C. Why?
Q.8 HI is the strongest halogen acid, whereas H–F is the weakest. Why?
Q.9 Wood pieces are used to hold ice-cream. Why?
Q.10 KHF2 is possible but not KHBr2 or KHI2. Why?
Q.11 O – Nitrophenol is less soluble in H2O than p – Nitrophenol. Why?
Q.12 o-Hydroxy benzaldehyde is a liquid at room temperature while p-hydroxy benzaldehyde is a
high melting solid.
Q.13 Glycerol is more viscous than ethanol. Explain.
Q.14 CH4 and H2O have nearly same molecular weight. Yet CH4 has a boiling point 112 K and
water has 373 K. Explain.
Q.15 The experimental molecular weight of acetic acid is just double than theoretical molecular
weight of acetic acid. Why?
Q.16 Although chlorine has same electronegativity as nitrogen but the former does not form
effective H-bonding. Explain.
Q.17 Molar entropy change of vapourization of acetic acid is less than that of water. Explain
Q.18 Heat of vapourization of water is higher than HF, however strength of H-bond in HF is higher
than water. Explain
You might also like
- Cade MY: Vineet Loomba UnacademyDocument2 pagesCade MY: Vineet Loomba UnacademyShivam RoyNo ratings yet
- Height & Distance Sheet by Om SirDocument7 pagesHeight & Distance Sheet by Om SirShivam RoyNo ratings yet
- Part - I: Objective Questions: Section (A) : Aromatic Electrolophilic Substitution Reaction (Ars 2)Document6 pagesPart - I: Objective Questions: Section (A) : Aromatic Electrolophilic Substitution Reaction (Ars 2)Shivam RoyNo ratings yet
- Kinametics (Rectilinear+Motion) Exercise-5 (B) +Document2 pagesKinametics (Rectilinear+Motion) Exercise-5 (B) +Shivam RoyNo ratings yet
- ZTM Nmap Cheatsheet Version 1 01Document13 pagesZTM Nmap Cheatsheet Version 1 01Shivam RoyNo ratings yet
- DPP Optical+Isomer 4Document2 pagesDPP Optical+Isomer 4Shivam RoyNo ratings yet
- DPP+2++Gravitation+07 01 2021Document7 pagesDPP+2++Gravitation+07 01 2021Shivam RoyNo ratings yet
- 400+ Love Shayari in English - Romantic, Sad, Girlfriend & BoyfriendDocument65 pages400+ Love Shayari in English - Romantic, Sad, Girlfriend & BoyfriendShivam RoyNo ratings yet
- Dpp-5 (Binomial Theorem)Document1 pageDpp-5 (Binomial Theorem)Shivam RoyNo ratings yet
- Assignment 1Document3 pagesAssignment 1Shivam RoyNo ratings yet
- Best Approach: Conic Section (Sheet)Document79 pagesBest Approach: Conic Section (Sheet)Shivam RoyNo ratings yet
- DPP-03&04 (Binomial Theorem)Document5 pagesDPP-03&04 (Binomial Theorem)Shivam RoyNo ratings yet
- Conic+Section WB-1Document69 pagesConic+Section WB-1Shivam RoyNo ratings yet
- Work Book HyperbolaDocument8 pagesWork Book HyperbolaShivam RoyNo ratings yet
- DPP 54+conicDocument3 pagesDPP 54+conicShivam RoyNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)