Professional Documents
Culture Documents
Morphological Changes
Uploaded by
Shah NawazCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Morphological Changes
Uploaded by
Shah NawazCopyright:
Available Formats
Morphologic changes in viral infections
1. Morphological Changes in Viral Infections Shridhan Patil 25 June 2012
2. "The Father of Modern Vaccines." Nobel laureate John Franklin Enders • Known for polio viruses •
Classified viruses on the basis of morphologic effects on cells.
3. Virus NOT considered as cells • Small, obligate intracellular parasites • Strucure – DNA/RNA and protein
coat • Mobile genetic elements • Virion - Function
4. Classification • Size and shape • Chemical composition • Structure of genome • Modes of replication
5. Cytopathic effect(CPE) • Morphological changes in cells caused by viral infections; the responsible virus is
said to be cytopathogenic • Degree of CPE depends on o Virus and host type o Multiplicity of infection
(MOI) o Some viruses do not cause CPE – Interference/ hemadsorption • Application- Virus identification,
unknown virus
6. How virus infects • Live inside cells- avoid immune response • Attachment receptors- DOCKING •
PENETRATION- cytoplasm • BIOSYNTHESIS of viral components • MATURATION • RELEASE
7. Sampling • What types of cases): stool, tissue, specimens are • Gastrointestinal tract saliva, brain biopsy,
collected to diagnose? infections: cerebrospinal fluid stool and rectal swabs • Respiratory tract • Genital
infections: infections: • Vesicular rash: vesicle vesicle fluid or Nasal fluid, skin Swab and bronchial Scrapings
washings, throat • Urinary tract infections: and nasal swabs, sputum • Maculopapular rash: urine throat, stool,
• Eye infections: throat and rectal swabs • Bloodborne infections: and blood • conjunctival • CNS
(encephalitis and swab/scraping meningitis
8. Techniques of virus study • Direct 1. Electron microscopy/ Immuno-electroon microscopy 2. Light
microscopy 3. Ag detection, molecular methods etc.. • Indirect 1. Cell culture 2. Egg pocks on chorio-
allantoic membrane 3. Animal disease/death
9. • Find suitable host – primary, semi-continuous, continuous cells • Host grown as MONOLAYER on a solid
surface • Infect cell with virus of interest- ONE VIRION/cell • Circle of death- easily counted and quantified
10. Cytopathic effects • Clinical signs, symptoms- morphologic- physiologic- biochemical- immunologic
effects of viruses • Unfixed, unstained smears- 10x- some CPE directly observed • Translucent cells-
condenser down- iris diaphragm partly closed • Inclusion bodies fixation and staining
11. • Requires experience to use CPE as diagnostic tool • Virus may not conform to norm for its family • CPE
best viewed by daily observation of cultures admixed with low MOI (<0.1) • Normal cell ageing to be
distinguished • Rate of CPE appearance- characteristic • General rule : Slow virus- 4-5 days • Rapid – 1-2
days for CPE to appear
12. Types 1) Total destruction o Of all monolayer- most severe o Cells shrink rapidly, become pyknotic o
Detach from glass within 72 hrs o Typical of ENTEROVIRUSES 20x objective within 24 hrs Bovine fetal
spleen cells
13. 2) Subtotal Bovine fetal spleen cells 2 destruction days • Some cells postinfection dead/detached
Rhabdovirus • Togavirus • Some picornavirus • Paramyxovirus 20x objective
14. 3) Focal degeneration • Herpesvirus, Poxvirus • Localised foci of infection • D/t cell to cell virus trasnfer
rather than extracellularly • Enlarged, rounded, refractile cells Bovine fetal spleen cells 2 days postinfection,
Herpesvirus • Strangling of cytoplasm Black arrows - cell rounding in a focal pattern. • Cell fusion Blue
arrows -cytoplasmic stranding. 20x objective . IMP: view at a low MOI
15. 4) Swelling and clumping • Adenovirus • Cells greatly enlarge and clump together in ‘grape-like’ clusters
Bovine fetal spleen cells 4 days postinfection with a high MOI of the bovine adenovirus, an Adenovirus,
showing cell rounding and small amounts of clumping. 10X objective
16. 5) Foamy degeneratioon • d/t large/numerous cytoplasmic vacuoles • Retrovirus • Paramyxovirus •
Flavivirus • Difficult to see without Giemsa-stained bovine fetal spleen cells 1 day staining Flavivirus, showing
vacuoles (arrow). 40X objective
17. 6) Cell fusion • Syncytium/polykaryon • Plasma membranes of 4 or more cells fuse • Enlarged cell with 4
or more nuclei • Easily seen when stained • To be distinguished from cell Giemsa-stained bovine fetal
clumping/clustering spleen cells 4 days • Paramyxovirus postinfection with the • Herpesvirus bovine
respiratory syncytial virus, a Paramyxovirus, showing syncytia (arrows) and faint basophilic cytoplasmic
inclusion bodies (dashed arrows). 20x objective
18. 7) Inclusion bodies • Areas of altered cell staining • CANNOT be seen in live cell cultures • Single or
multiple- depends on virus • Large/small • Round/irregular • Intranuclear/intracytoplasmic •
Eosinophilic/basophilic • Chromatin margination • Indicate areas of viral protein synthesis or nucleic acid
synthesis or virion assembly • Some cases no virus is present Herpesvirus, 40x. Viral scarring
19. Cytoplasmic stranding Adenovirus, 20x. Intranuclear inclusion Rough-edged nuclear inclusion bodies
20. Poxvirus, showing pink eosinophilic cytoplasmic Feline nasal turbinate primary cell culture infected
inclusion bodies (arrows) and cell swelling near the with feline herpes virus-1, a Herpesvirus, showing top of
the field, 20x. chromatin margination (dark ring around the edge of the nucleus).
21. CPE of Common Viruses • MEASLES o dilated skin vessels, edema o Lymph nodes, lungs o Warthin-
Finkeldey cells
22. • MUMPS o Parotitis o Orchitis o Pancreas o Rarely encephalitis Mumps parotitis Enlarged glands, C/S
reddish brown, Glistening. Macrophage, lymphocyte infiltration. Oedematous
23. • HERPES SIMPLEX o Fever blisters o Gingivostomatitis o Genital herpes o Herpes epithelial keratitis o
Herpes stromal keratitis o Cowdry type A inclusion bodies • Pink to purple intranuclear inclusions Glassy
intranuclear herpes simplex inclusion bodies
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Equipment Maintenance and Measuring Equipment ProcedureDocument2 pagesEquipment Maintenance and Measuring Equipment ProcedureRaja Mani100% (1)
- ADAPT-Builder 2019 GUI Quick Reference GuideDocument103 pagesADAPT-Builder 2019 GUI Quick Reference GuideephremNo ratings yet
- Orange County Sheriff's Office SeaWorld Death Investigative ReportDocument43 pagesOrange County Sheriff's Office SeaWorld Death Investigative ReportWESH2News100% (1)
- Equipments Used in PelletingDocument3 pagesEquipments Used in PelletingShah NawazNo ratings yet
- Essential Oils As A Feed Additive in PouDocument7 pagesEssential Oils As A Feed Additive in PouShah NawazNo ratings yet
- Job Interviews: Mr. Cowan Futures Forum FhciDocument9 pagesJob Interviews: Mr. Cowan Futures Forum FhciShah NawazNo ratings yet
- Job Search & Interview Skills: or Some Real-World Advice That May Prove Useful To YouDocument97 pagesJob Search & Interview Skills: or Some Real-World Advice That May Prove Useful To YouShah NawazNo ratings yet
- Deletarious Substances in Poultry FeedDocument4 pagesDeletarious Substances in Poultry FeedShah NawazNo ratings yet
- Interviewing For A Job: Preparing For The InterviewDocument20 pagesInterviewing For A Job: Preparing For The InterviewShah NawazNo ratings yet
- Atlas of Avian Diseases - Partners in Animal HealthDocument2 pagesAtlas of Avian Diseases - Partners in Animal HealthShah NawazNo ratings yet
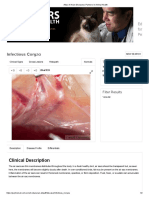
- Ed For Pet: Clinical DescriptionDocument2 pagesEd For Pet: Clinical DescriptionShah NawazNo ratings yet
- Contribution of Meat Inspection To The Surveillance of Poultry Health and Welfare in The European UnionDocument14 pagesContribution of Meat Inspection To The Surveillance of Poultry Health and Welfare in The European UnionShah NawazNo ratings yet
- Gut Health and Immunity Key in Poultry Production - Pashudhan PrahareeDocument3 pagesGut Health and Immunity Key in Poultry Production - Pashudhan PrahareeShah NawazNo ratings yet
- Ed For Pet: Clinical DescriptionDocument2 pagesEd For Pet: Clinical DescriptionShah NawazNo ratings yet
- Avian Encephalomyelitis (AE)Document23 pagesAvian Encephalomyelitis (AE)Shah NawazNo ratings yet
- Ed For Pet: Clinical DescriptionDocument2 pagesEd For Pet: Clinical DescriptionShah NawazNo ratings yet
- Ed For Pet: Clinical DescriptionDocument2 pagesEd For Pet: Clinical DescriptionShah NawazNo ratings yet
- HATCHING EGG COLLECTION & CARE - Pashudhan PrahareeDocument3 pagesHATCHING EGG COLLECTION & CARE - Pashudhan PrahareeShah NawazNo ratings yet
- Effect of Broiler Breeders Age On HatchaDocument7 pagesEffect of Broiler Breeders Age On HatchaShah NawazNo ratings yet
- Avian Influenza: Communicable Disease Surveillance and Response, WHODocument21 pagesAvian Influenza: Communicable Disease Surveillance and Response, WHOShah NawazNo ratings yet
- Zinc Deficiency in ChickensDocument6 pagesZinc Deficiency in ChickensShah NawazNo ratings yet
- MEASUREMENT OF SHELL AND EGG QUALITY OF POULTRY EGGS - Pashudhan PrahareeDocument6 pagesMEASUREMENT OF SHELL AND EGG QUALITY OF POULTRY EGGS - Pashudhan PrahareeShah NawazNo ratings yet
- White Muscle Disease in ChickensDocument5 pagesWhite Muscle Disease in ChickensShah NawazNo ratings yet
- What Does It All MeanDocument16 pagesWhat Does It All MeanShah NawazNo ratings yet
- Avian Influenza A (H5N1)Document15 pagesAvian Influenza A (H5N1)Shah NawazNo ratings yet
- Zinc Toxicity in ChickensDocument6 pagesZinc Toxicity in ChickensShah NawazNo ratings yet
- Anatomical Pathology and Histopathological ChanDocument9 pagesAnatomical Pathology and Histopathological ChanShah NawazNo ratings yet
- Avian Influenza - The BirdDocument13 pagesAvian Influenza - The BirdShah NawazNo ratings yet
- The Role of Vaccination & Lab Monitoring in The Control of Poultry Diseases.Document35 pagesThe Role of Vaccination & Lab Monitoring in The Control of Poultry Diseases.Shah NawazNo ratings yet
- Spongy Bone Histology - Bony Trabeculae and Marrow Space Structure AnatomyLearner - The Place To Learn Veterinary Anatomy OnlineDocument14 pagesSpongy Bone Histology - Bony Trabeculae and Marrow Space Structure AnatomyLearner - The Place To Learn Veterinary Anatomy OnlineShah NawazNo ratings yet
- Finite Element Modeling Analysis of Nano Composite Airfoil StructureDocument11 pagesFinite Element Modeling Analysis of Nano Composite Airfoil StructureSuraj GautamNo ratings yet
- თინათინ ზურაბიშვილი, თვისებრივი მეთოდებიDocument111 pagesთინათინ ზურაბიშვილი, თვისებრივი მეთოდებიNino LomaiaNo ratings yet
- Spies May Be Gathering Encrypted Data To Crack With Future Quantum ComputerDocument1 pageSpies May Be Gathering Encrypted Data To Crack With Future Quantum ComputerHÀ ĐỖ VIẾTNo ratings yet
- NEW Sample ISAT Questions RevisedDocument14 pagesNEW Sample ISAT Questions RevisedHa HoangNo ratings yet
- Compal Confidential: Ziwb2/Ziwb3/Ziwe1 DIS M/B Schematics DocumentDocument56 pagesCompal Confidential: Ziwb2/Ziwb3/Ziwe1 DIS M/B Schematics DocumentSuhpreetNo ratings yet
- Ofsaai Ic 72 E22351 01Document312 pagesOfsaai Ic 72 E22351 01Mohamed AbrarNo ratings yet
- Organic Food Business in India A Survey of CompaniDocument19 pagesOrganic Food Business in India A Survey of CompaniShravan KemturNo ratings yet
- Best S and Nocella, III (Eds.) - Igniting A Revolution - Voices in Defense of The Earth PDFDocument455 pagesBest S and Nocella, III (Eds.) - Igniting A Revolution - Voices in Defense of The Earth PDFRune Skjold LarsenNo ratings yet
- Auditing BasicsDocument197 pagesAuditing BasicsMajanja AsheryNo ratings yet
- 2 MercaptoEthanolDocument8 pages2 MercaptoEthanolMuhamad ZakyNo ratings yet
- 1991 Hanaor - DOUBLE-LAYER TENSEGRITY GRIDS - STATIC LOADDocument15 pages1991 Hanaor - DOUBLE-LAYER TENSEGRITY GRIDS - STATIC LOADDaniel MartinsNo ratings yet
- Peter Brandt InterviewDocument38 pagesPeter Brandt InterviewNishant P Kalaskar100% (1)
- House Staff OrderDocument2 pagesHouse Staff OrderTarikNo ratings yet
- The Roti Canai StoryDocument5 pagesThe Roti Canai StoryDr Bugs TanNo ratings yet
- Periodicity Review SL KeyDocument4 pagesPeriodicity Review SL KeyYeyoung ParkNo ratings yet
- BROMINE Safety Handbook - Web FinalDocument110 pagesBROMINE Safety Handbook - Web Finalmonil panchalNo ratings yet
- Test Bank Bank For Advanced Accounting 1 E by Bline 382235889 Test Bank Bank For Advanced Accounting 1 E by BlineDocument31 pagesTest Bank Bank For Advanced Accounting 1 E by Bline 382235889 Test Bank Bank For Advanced Accounting 1 E by BlineDe GuzmanNo ratings yet
- Transformational Leadership in The UmcDocument17 pagesTransformational Leadership in The Umcapi-202352366No ratings yet
- Dr. N. Kumarappan IE (I) Council Candidate - Electrical DivisionDocument1 pageDr. N. Kumarappan IE (I) Council Candidate - Electrical Divisionshanmugasundaram32No ratings yet
- Dell Inspiron 5547 15Document7 pagesDell Inspiron 5547 15Kiti HowaitoNo ratings yet
- Refrigerant Unit Lab ReportDocument19 pagesRefrigerant Unit Lab Reportakmal100% (2)
- Week 1 Macro (DDR)Document49 pagesWeek 1 Macro (DDR)Stevie Sean100% (1)
- Coastal Blue Carbon - Methods For Assessing Carbon Stocks and Emissions Factors in Mangroves Tidal Salt Marshes and Seagrass MeadowsDocument182 pagesCoastal Blue Carbon - Methods For Assessing Carbon Stocks and Emissions Factors in Mangroves Tidal Salt Marshes and Seagrass Meadowsapi-245803001No ratings yet
- Decs vs. San DiegoDocument7 pagesDecs vs. San Diegochini17100% (2)
- AnnexIIRecommendationsbyHOTCCommittee06 11 18Document6 pagesAnnexIIRecommendationsbyHOTCCommittee06 11 18Bilal AbbasNo ratings yet
- Ias Book 2015Document49 pagesIas Book 2015Rahul SharmaNo ratings yet