Professional Documents
Culture Documents
IR-Infrared Thermography Level III
IR-Infrared Thermography Level III
Uploaded by
vignesh seeniraj0 ratings0% found this document useful (0 votes)
44 views117 pagesCopyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
44 views117 pagesIR-Infrared Thermography Level III
IR-Infrared Thermography Level III
Uploaded by
vignesh seenirajCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 117
IR-Infrared Thermography Level III
Chapter 1
Principles / Theory
Introduction to Principles and Theory
& Infrared/thermal testing involves the use of temperature and heat flow measurement as
a means to predict or diagnose failure. This may involve the use of contacting or
noncontacting devices, or a combination of both.
+ Contacting devices include:
a) thermometers of various types,
b) thermocouples,
©) thermopiles and
d) thermochromic coatings.
4 Noncontacting devices include:
a) convection (heat flux) devices,
b) optical pyrometers,
©) infrared radiation thermometers,
4) infrared Linescanners and
©) infrared thermal imaging (thermographic) equipment.
& Infrared thermography is
> the nondestructive, non-intrusive, noncontact mapping of thermal patterns on the
surface of objects.
> Itis usually used to diagnose thermal behavior and, thereby, to assess the
performance of equipment and the integrity of materials, products and processes.
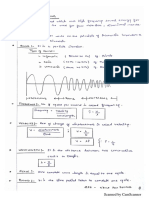
& The thermal maps “Thermal image” produced by infrared thermal imaging instruments
are called thermogeams.
> Inthe thermograms, temperatures are displayed in a spectrum of colors.
ig Hierarchy of colors
1] Page
> This chart illustrates the hierarchy of colors used to represent the relative temperature
differences of the problems found during the inspection,
& To understand and interpret thermograms, the thermographer must be familiar with
a) the fundamentals of temperature and heat transfer, infrared radiative heat flow and
b) the performance of infrared thermal imaging instruments and other thermal
instruments.
Fundamentals of Temperature and Heat Transfer
bHeat
‘Heat may be defined as a form of energy created by the molecular motions of an
object.
+> Heat, unlike temperature, is a measure of the totallkinetie energy of all the
the objects.
+ Asa form of energy, heat has the unit joule (J)in the International System of Units (SI).
molecule:
However, in many applied fields in engineering the British thermal unit (BTU) and the calorie
are often used. _ Heat = Mass x (Te)
> Calor
YA calorie will raise 1 gram of water 1 degree Celsius.
> — joule
Is the quantity of heat needed to raise the temperature of 1 g of water by 1/4.184°C.
> BT
¥ ABritish Thermal Unit (BTU) is a measurement of heat energy. One BTU is the amount
of heat energy required to raise one pound of water by 12F,
Example 1)
Pretend that you have 10 grams of water at 70 degrees Celsius. How many calories are
needed to get the sample to boiling? (Bolling occurs at 100 degrees Celsius)
Heat = Mass X (T2-T1)
Heat = 10 x (100-70)
Heat = 300 calories
Example 2)
You have 200 grams of water at 65 degrees Celsius. If you apply 2000 calories to the
sample, what will the final temperature be?
Heat = Mass X (T2-T1)
2|Page
2000 = 200 x (12-65)
T2=759C
Specific Heat
‘The specific heat is the amount of heat per unit mass required to raise the
temperature by one degree Celsius.
“The relationship between heat and temperature change is usually expressed in the
form shown below where C is the specific heat.
Q =C M aT
> where,
¥ Q isheatadded, Joule
v Cis heat specific heat, Joule per kelvin
v Misheat mass, kilogram
v_ Tisheat change in temperature, kelvin
Heat capacity or thermal capacity,
“The heat capacity of a material or structure describes its ability to store heat.
‘isthe product ofthe ied BE SERERY Io) RIE GRE
‘This means that denser materials generally will have higher heat capacities than
porous materials.
Heat Capacity volumetric = Cp. 0
> Where,
¥ WY AT ks Heat copscny, ue permet
¥ CP isheat specific heat, Joule per kelvin
3|Page
Y The heat capacity of an object Q/AT. is directly proportional to a material's
specificheat C anddensity 0
+ Heat flow/transfer
‘Heat flow is thermal energy in transit and heat always flows from warmer objects to
cooler objects.
& Temperature
‘Temperature is a measure of the thermal energy contained by an object; the degree
of hotness or coldness of an object (e.g. atmosphere, living body) measurable by any
of a number of relative scales.
& Ameasure of the quantity of heat present in something.
4 Modes of Heat Transfer
‘The three modes of heat transfer are conductive, convective and ra
+ Allheat is transferred by one of these three modes.
In most situations, heat is transferred by a combination of two or all three modes.
Infrared thermography is most closely associated with the radiative process, but it is essential
to study all three to understand the meaning of #hehiniOgraiis and to pursue a successful
program of thermography.
Temperature and Temperature Seales
& Temperature is expressed in either absolute or relative terms.
“ Thete are two absolute scales called
> Rankine (English system) and
Kelvin (metric system).
‘There are two corresponding relative scales called
> Fahrenheit (English system) and
> Celsius or centigrade (metric system}
4 Relative temperature is expressed as degrees Celsius or degrees Fahrenheit (°C or °F).
& The numerical relations among the four scales are as follows:
“ Tcelsius = 5/9 (Tfahrenheit - 32)
4|Page
“© Tfahrenheit = (9/5 T celsius) + 32
“ TRankine = T fahrenheit + 459.7
© TKelvin = T+ 273.16
+ Example 1)
> You have found a motor bearing that is 32 °F warmer than normal. Convert this
temperature to degrees Celsius.
T celsius = 5/9 (Tfahrenheit - 32)
T celsius = 5/9 (32 - 32)
Tcelsius =0 °C
To onvettia ehaiigelin temperature or Helta T (AMT) between the English and metric
systems, the simple 8/5\(U18t0i1) relationship is used:
> AT Fahrenheit (or ° Rankine) = 1.8% AT Celsius (or Kelvin)
+ Example 2)
> You have found a motor bearing that is 32 °F warmer than normal. Convert this
temperature difference (AT) to degrees Celsius.
AT Fahrenheit = 1.8x AT Celsius
AT Celsius = AT Fahrenheit /1.8
AT Celsius = 32 /1.8
AT Celsius = 17.7 °C
& Absolute zero
Absolute zero is the temperature at which no molecular action takes place,
+ Absolute zero is expressed as HBPOIKGIVIN or ZEPSUEEFEESIRERKIN.
Absolute zero is equal to {12736 and also equal to approximately SASSI7EF.
+ All bodies whose temperature is at the absolute zero point, emit 6 infrared
radiation.
273.15 °C) emits
Every object with a temperature aBOVE absolute zero (0 Kelvin
Infrared radiation.
5|Page
Laws of thermodynamics
“The four laws of thermodynamics are
0) Zeroth law of thermodynamics.
ob The law states “IF two thermodynamic systems are each in thermal equilibrium with a
third, then they are in thermal equilibrium with each other.”
> Basically, if A=B and C=B then A=C. This may seem so obvious that is doesn’t need
stating but without this law we couldn't define the concept of temperature and we
couldn’t build thermometer.
1) First law of thermodynamics
“& The law of conservation of energy.
> The law states “that energy can be neither created nor destroyed. However, energy can
change forms, and energy can flow from one place to another”.
> In any process, the total energy of the universe remains the same, For a
thermodynamic cycle the net heat supplied to the system equals the net work
done by the system.
> Aparticular consequence of the law of conservation of energy is that the total energy of an
Isolated system does not change (is constant).
> The flow of heat
a form of energy transfer.
> Heating is’a natural process of moving energy to or from a system other than by
work or the transfer of matter.
“4 The first law is often formulated by stating that the change in the internal energy of a
closed system U is equal to the amount of heat supplied to the system Q, minus the
amount of work done by the system on its surroundings W.
U=Q-W
2) Second law of thermodynamics
+ One statement for the Second Law relating to heat:
> When ahot and a cold body are brought into contact with each other, heat
ergy ill ow from tie hot body tolRhe EOIA Bady until they reach thermal
equilibrium, i.e., the same temperature. However, the heat will never move
6 [Page
back the other way; the difference in the temperatures of the two bodies will
never spontaneously increase,
> Moving heat from a cold body to a hot body requires work to be done by an
external energy source such as a heat pump.
4 Work and energy
> the he Second Law explains is that itis impossible to convert heat energy to
mechanical energy with 100 percent efficiency.
4 The arrow of time
> The Second Law indicates that thermodynamic processes, i-e., processes that
involve the transfer or conversion of heat energy, are irreversible because they
all result in SAliMer@a8e In| EREFOpY.
4 The fate of the universe
> The total entropy of the Universe increases whenever a real process occurs.
Hence, the £8tal SHEFOpY of the Universe continually increases. Entropy
increases, energy becomes less available, and the universe becomes more
random or more “run down”.
> The Second Law also predicts the end of the universe, according to Boston
University. “It implies that the universe will end in a ‘heat death’ in which
everything is at the same temperature. This is the ultimate level of disorder; if
everything is at the same temperature, no work can be done, and all the energy
will end up as the random motion of atoms and molecules,
> Atransformation whose only final result is to convert heat, extracted from a
source at constant temperature, into work, is impossible,
4 Entropy “as”
>a measure of the unavailable energy in a closed thermodynamic system that is
also usually considered to be a measure of the system's disorder, that is a
property of the system's state, and that varies “AS” directly with any reversible
change in heat in the system” Q “and inversely with the temperature of the
system “T",
aS=Q/T
> Entropy “AS” is a measure of the degree of disorder or uncertainty in a system.
> The Processes that are not reversible are called irreversible.
3) Third law of thermodynamics
“4 The third law provides an absolute reference point for measuring entropy, saying
that
T|Page
> As the temperature ofa system approaches HSRESSR=TSAETENSTR] the
ERRFOBY of a system approaches B/ebnstanit/ minimum Value,
Conductive Heat Transfer
Itis the transfer of beat in stationary media. It is the only mode of heat flow in solids, but
it can also take place in liquids and gases. It is usually thought of as a very slow process in
the case of gases.
4 Conductive heat transfer occurs as the result of atomic vibrations (in solids) and molecular
collisions (in liquids) whereby energy is moved, one molecule at a time, from higher
temperature sites to lower temperature sites.
& An example of conductive heat transfer is when one end of alsection of metal pipe warms
up after a flame is applied to the other end.
& The Fourier Law
“The law of heat conduction, also known as Fourier's law, states that “the time rate of
heat transfer through a material is proportional to the negative gradient in the
‘temperature and to the area, at right angles to that gradient, through which the heat
flows”,
“We can state this law in two equivalent forms:
> the integral form, in which we look at the amount of energy flowing into or out
of a body as a whole, and
v
the differential form, in which we look at the flow rates or fluxes of energy
locally.
Newton's law of cooling is a discrete analogue of Fourier's law, while Ohm's law is
the electrical analogue of Fourier's law. :
+) Fourier’s equation of heat conduction:
Q=-kA(dT/dx)
> Where,
Y ‘Qis the heat flow rate by conduction (W or J/s)
“K’is the thermal conductivity of body material (W/m-K)
¥
Y ‘R'is the cross-sectional area normal to direction of heat flow (m?) and
Y ‘dT/dx is the temperature gradient (K/m).
B|Page
> Negative sign in Fourier’s equation indicates that the heat flow is in the direction
of negative gradient temperature and that serves to make heat flow positive,
v
di
the rate of heat conduction through a layer is proportional to the temperature
ference across the layer and the heat transfer area, but itis inversely
proportional to the thickness of the layer. In real terms, the Fourier expression
means that the rate of heat flow increases with increasing temperature
difference, increases with increasing thermal conductivity and decreases with
Increasing slab thickness.
> Thermal Conductivity
v
Thermal conductivity K [W/M. K] is a measure of a material's ability to
conduct heat and The material property that relates to the rate that heat
flows through a solic
The thermal conductivity is defined as the rate of heat transfer through a unit
thickness of material per unit area per unit temperature difference.
Materials with high thermal conductivities are high heat EORGUCtORS.
‘An isotropic material is a material that has uniform properties in all
directions.
Insulators are materials used primarily to provide resistance to heat flow.
They have low thérmal conductivity.
> The Thermal Resistarice Concept
9|Page
‘The Fourier equation, for steady conduction through a constant area plane
wall, can be written: d T, -T,
o ~~~ 14
dx L
‘A. Q/A Heat flow per unit area (W/m?) is defined as:
2 % —T;
Zz
This can be re-arranged as:
B. RIK /W]is the thermal resistance of the wall against heat conduction
or simply the conduction resistance of the wall.
C. Thermal conductivity K [W/M. K] is defined as:
L
RA
+ Thermal concluetity Is highest for metals such afalliminum and loWer for porous
materials such as briek It's inversely proportional to theimal resistance (thermal
resistivity).
Example 1)
> Determine the steady state rate of heat transfer per unit area through a 4.0cm
thick homogeneous slab with its two faces maintained at uniform temperatures
The thermal conductivity of the material is 0.19 W/m K.
2 .,~,i-h
Q/A = 0.19 (W/m. K) [ (38-21) °C /0.04 M
Q/A =80.75 W/m?
Example 2)
> Calculate the thermal resistance and the rate of heat transfer through a pane of,
‘window glass (k = 0.78 W/m k) 1m high, 0.5 m wide, and 0.5 cm thick, if the
outer-surface temperature is 24°C and the inner-surface temperature is 24.5°C
pee 0.005m oon =
kA 0.78w/ mk x lm x 0.5m v
The rate of heat loss from the interior to the exterior surface is
AT _ 245-24
10 [Page SR 00128
Convective Heat Transfer
+ Convective heat transfer takes place in a moving medium and is almost always associated
with heat transfer between a solid and a moving fluid (such as air).
+ In convective heat flow, heat transfer takes effect by direct conduction through the fluid
and the mixing motion of the fluid itself.
-& Whenever a solid body is exposed to a moving fluid having |” ,. t=) 1
[My Crow
a temperature different from that of the body, i
energy is carried or convected from or Fa velcy Temperature
Fy isaton
to the body by the fluid If the 0)
ar 1,
esses! a
the surface temperature of the solid is Ts, thelheat at) Heed sry)
transfer per unit time is given by Newton's Lawof cooling '
4 Newton's law of cooling
“Newton's law of cooling states that the rate of heat loss of a body is directly
upstream temperature of the fluid is Te», and
locity and temperature distribution on fla plate
proportional to the difference in the temperatures between the body and its
surroundings provided the temperature difference is small and the nature of radiating
surface remains same,
4 Newton's cooling law in convection isa restatement of the differential equation
given by Fourier's law:
dQ/dt = h-A(Ts —T.)
> Whére,
YQis the heat flow rate by convection, (W or J/s)
vf isthe (convective) heat transfer coefficient, W/ (m? K)
vA isthe unit surface area of the body through which the heat is transferred, m?
v_ Tsis the temperature of the surface of the body (solid), K
¥ Too isthe temperature of the surroundings (fluid), K
‘It is important to keep in mind that the fundamental energy exchange at a solid-fluid
boundary is by ohduetiOn, and that this energy is then converted away by the fluid
flow.
A. Q/A Heat flow per unit area (W/m?) is defined as:
Q/A =h- (Ts -T.)
B. Convective Heat transfer coefficient (h) (W/m? K) as the constant of
proportionality relating the heat transfer per unit time and area to the
overall temperature difference.
h=Q/ A. (Ts To)
C. The thermal resistance to convection heat transfer R (K/W), as:
2=
<> Rils@asier toluselthanlh when determining combined conductive and convective heat
transfer because then they are additive terms.
“The rate of convective heat flow increases with
> increasing temperature difference,
> increases with higher convective heat flow coefficient and
+P) The rate of convettive heat flow decreases with
> Increasing convective thermal resistance.
“Conductive and convective heat transfer are very similar.
> In both, the heat transfer is directly proportional to the temperature
difference and the speed at which this energy is transferred (rate of heat flow)
depends on the transfer coefficient of the media or material through which the
heat energy flows.
> Example 1)
> The forced convective heat transfer coefficient for a hot fluid x1 x2 flowing over
a cool surface is 225 W/m. °C for a particular problem. The fluid temperature
upstream of the cool surface is 120 C, and the surface is held at 10 °C. Determine
the heat transfer rate per unit surface area from the fluid to the surface.
Q /A= h (Ts-Tee)
Q/A= 225 (120-10) =24750 W/m?
Radiative Heat Transfer
+ Radiative heat transfer is unlike the other two modes because:
1) it occurs by electromagnetic emission and absorption ina manner similar to light;
2) it propagates at the speed of light;
3) like light, it requires a direct line of sight;
4) the heat energy transferred is proportional to {HefOUrtN POWEFT! of the temperature
of the objects; and
5) it can take place across a vacuum — in fact, a vacuum is the most efficient medium for
radiative heat transfer.
+ The Stefan-Boltzmann law!
+ The Stefan—Boltzmann law describes the power radiated from a black body in terms of
its temperature,
+ Specifically, the Stefan—Boltzmann law states that the total energy radiated per unit
surface area of a blackbody across all wavelengths per unit time
4 also known as the black-body radiant emittance is directly proportional to the fourth
power of the black body's thermodynamic temperature T:
Q/A = 6 Tt
> Where,
¥ Qis the total energy radiated, (W or J/s)
¥ T isthe absolute temperature, (K}
¥ @ is Boltzmann constant independent of surface, medium, and temperature;
> the thermal emission from many surfaces (gray bodies) can be well represented
by
Q /A= o & T*
Q /A= o & (Ts*-Tsur*)
= Where
¥- &, the emissivity of the surface, ranges (0-1). The ideal emitter or blackbody
is one, All other surfaces emit somewhat less than one.
¥ Ts and Tyur, the temperature of surface and surroundings respectively.
¥ Similarly, The thermal resistance to radiation heat transfer Ri, as:
‘> Example 1)
> After sunset, radiant energy can be sensed by a person standing near a brick
wall. Such walls frequently have surface temperatures around 44 °C, and typical
brick emissivity values are on the order of 0.92. What would be the radiant
thermal flux per square meter from a brick wall at this temperature?
Q/A= 06 Tt
Q/A = 0.92 x 5.6697 x 10°(44+237)*
Q/A = 527 w/m?
aa] Page
& Table 1 Summary of heat transfer rate processes
Mode Tonio Mebane Rate of | a ‘transfer cia eee
Diffusion of energy due to
random molecular motion
kat
Conduction
Diffusion of energy due to
Convection random molecularmotion —-g = hh A(T,-T,)
plus bulk motion
Energy transfer by
ie cic
Radiation electromagnetic waves q = 0 ATS -Toy") aeMT 1)
N68! The concept of thermal resistance (analogous to electrical resistance) is introduced as an aid
‘to solving conduction heat transfer problems,
& Radioactive heat transfer takes place in the infrared portion of the spectrum, from
0.75ym to about 104m. Most instruments used in infrared thermography operate
somewhere within the 2t@/M@imH spectral region.
“& Most imaging infrared radiometers operate in the Byam te Sum OF pm tO A2jaM band. This
is because of atmospheric absorption within these two bands is small enough to provide
minimal impact on radiometry.
Xerays | Uttra- intrared | Radio
omic \
3 eHe SH UME VHE He Me UF vir
hens cee SD admaaianl T_T
0.01 04 140 100-04 130 400
nm fm pin jim him em km tom km
-- ‘Wavelength
Intrared Measurement Region
04 075 10 1520 30 50 10 20 30
‘Wavelength (umm)
4 The measurement unit for radiant energy wavelength
> Armicron (um) is BRe=INGREH ofa meter
> Ananometer (nm) is Bie billionth ofa meter.
> Wavelength is inversely related to frequency (longer wavelengths have lower
frequencies).
15 Page
Fundamentals of Radiative Heat Flow
4 To understanding the Fundamentals of Radiative Heat Flow you must know the following
definitions: -
‘% Transmissivity, (transmittance) ( t ).
= The proportion of infrared radiant energy impinging on an object's surface. For any given
spectral interval that is transmitted through the object; t= 1-€-p
* Fora blackbody, transmissivity ( ¢ )= 0.
+ Transmissivity is the internal transmittance per unit thickness of a non-dlffusing material.
% Reflectivity, (reflectance) (p)
* The ratio of the total energy reflected from a surface to total incidence on thatsurface; p=
Le-t
* pfora perfect mirror this approaches 1.0” that's mean a perfect reflector and Poor
Emitter",
= pfora blackbody the reflectivity is 0.
= Technically reflectivity is the ratio of the intensity of the reflected radiation to the total
radiation and reflectance is the ratio of the reflected flux to the incident flux. In
thermography, the two terms are often used interchangeably.
+ Exitance, radiant (also called radiosity)
"Total infrared energy (radiant flux) exitance leaving a target surface. This is composed of
radiated, reflected and transmitted components. Only the radiated components related to
target surface temperature)
“Emissivity, (Emittance) (e)
= Emissivity isa measure of the ability of a material to emit (give off) infrared radiation and itis
the ratio of a target surface's radiance to that of a blackbody at the same temperature,
viewed from the same angle and over the same spectral interval; €= 1-p-«
‘* agenericlookup value for a material Values range from 0 to 1.0,
| Aperfect emitter is referred to as a Blackbody, = 1, that's mean (Poor Reflector)
= All bodies in nature are colored bodies and have an ¢ <1.
= Agood emitter is a good absorber of energy.
+ Absorptivity, (absorptance) (a)
"The proportion (as a fraction of 2) of the radiant energy impinging on a material's surface
that is absorbed into the material.
= fora blackbody is unity (1.0).
= Technically, absorptivity is the internal absorptance per unit path length. In thermography,
the two terms are often used interchangeably.
16 [Page
+ As per Kirchhoff's Law For an arbitrary body emitting and absorbing thermal radiation in
thermodynamic equilibrium, the emissivity is equal to the absorptivity; 22a
4 Radiation Exchange at the Target Surface
+ The measurement of infrared/thermal radiation is the basis for non-contact
temperature measurement and infrared thermography.
* The surface to be evaluated is called the target surface.
+ Thermal infrared radiation leaving a surface is called Bxitanes OF radiosity.
“ It can be emitted from the surface, feflected by the surface, oF transmitted
through the surface.
illustrated in Figure.
Figure: Infrared radiation leaving a target surface I,
vy
1 al
e
Sv # .
e .
Reflected Radiation (W,)
mitted Radiation (W,)
ransmitted Radiation (W9
Target Surface
Wp= Gee T,!
oWe + SOW, + %p= 100% Wy Ger TA
W=oet TA
Wa + W,+ Wp= Target Exitance or Radiosity
47 [Pas
+ The total radiosity is equal to the sum of the emitted component (Wz), the reflected
component (W;) and the transmitted component (Wi).
+ Itisimportant to note that the surface temperature Te is related to the emitted
component We only.
+ Kirchhoff’s Law of thermal radfatior
> Thermal infrared radiation impinging on a surface can be absorbed, reflected, or
transmitted as illustrated in Figure below.
Figure: Infrared radiation impinging on
ste ee, pm Eat Bp + Be
> The sum of the three components is always equal to the total received radiation, Ey
the fractional sum of the three components equals unity or 100 percent.
Ex= Eat Ep+ Er
> Where,
Y E:=total energy
Y As per Kirchhoffs Law For an arbitrary body emitting and absorbing thermal
radiation in thermodynamic equilibrium, the emissivity is equal to the
absorptivity.
emissivity « = absorptivity a
Likewise, the sum of the three material properties, transmissivity, reflecti
and emissivity, also always equals unity (1):
e+p+r=t
18| Page
Example 1)
Ifa surface has an emissivity of 0.35 and a reflectivity of 0.45. its transmissivity
would be:
ef pk zt i
t= p =e
t = 1-(0. 35+0. 45)
t= 0.20
> Example 2)
> The temperature of an aluminum bus bar is being measured. You have
determined emissivity is 0.15. What is the reflectivity of the bus bar?
® Who = 1
Mm “ele
8 ~al-“O. 15= 0.85
Example 3)
> The temperature of an aluminum tab is being measured. You have determined
reflectivity is 0.98. What is the emissivity of the bus bar?
et p =f
=F,
e = 1- 0.98= 0.02
19] Page
od Reflections off Specular and Diffuse Surfaces
* Specular Reflector
> Aperfectly smooth surface will reflect incident energy at an angle complementary
to the angle of incidence.
Specular Reflection
40°
eeeeeeernenewenemrerers ss .
50'
> When two angles add to 90°, we say they "Complement" each other.
> Example 1)
> What is the reflect angle of thermal radiation, if the angle of incidence thermal
radiation impinging on perfectly smooth surface is 55° degree?
@ = 90-.55= 35° degree
+ Diffuse Reflector
> Acompletely rough or structured surface will scatter or disperse all of the incident
nn uy
tsSeiSa!
|
“ No perfectly specular or perfectly diffuse surface can exist in nature, and all real
surfaces have some diffusivity and some specularity.
20 Page
+ These surface characteristics will determine the type and direction of the reflected
component of incident radiation.
+ When making practical measurements, the specularity or diffusivity of a target surface
are taken into account by compensating for the effective emissivity (€*) of the surface.
4 Radiant Energy Related to Target Surface Temperature
* All target surfaces warmer than absolute zero radiate energy in the infrared spectrum.
+ Figure:
> shows the spectral distribution of energy 10°
radiating from various idealized target
surfaces as a function of surface 10°
Semperatire (0) nnd semiclengahi(Al). Amax
Very hot targets radiate in the vi 107 | 3000K ungsten
as well, and our eyes can see this “iG8 | | I Lr sa
Wet
because they are sensitive to light.
v
The idealized curves shown in Figure are
for perfect radiators known as blackbodies.
Planck’s law
v
* describes the electromagnetic radiation
emitted by a blackbodyjin thermal
equilibrium at a definite temperature and
AL
* MEA
describes the distribution of energy BVEE 107 A ‘i
ie HEEL PN |
aa rn
temperature. \
* gives a distribution that peaks at a certain 10°
wavelength, the peak shifts to shorter } | | AN \"
1 LE NN
wavelengths for higher temperatures, and
4
the area under the curve grows rapidly 101040810 10 100 1,000
Wavelength in Micrometers (1m)
Relative Emissive Power
with increasing temperature.
"The emitted radiation varies continuously with wavelength.
= At any wavelength the magnitude of the emitted radiation increases with
increasing temperature,
> Stephen-Boltzmann Law
= determines the total blackbody emissive power, which is the sum of the radiation
emitted over all wavelengths.
2a[Page
* describes integrate area under the curve to derive the total radiant flux “power”
emitted per unit area.
* illustrates that W, the total radiant flux emitted per unit area of surface, (the area
under the curve) is proportional to the fourth power of the absolute surface
ity of the surface, €.
temperature T4, a numerical constant o, and the emi
W= o€T*
Where,
Wis Radiant Flux per Unit Area, W/m?
€ is emissivity, (unity for a blackbody target)
© is Stefan-Boltzmann constant
o =5.673 x 10° W/mK‘
Tis absolute temperature of target, K
= Example 1)
After sunset, radiant energy can be sensed by a person standing near a brick
wall. Such walls frequently have surface temperatures around 44°C, and typical
brick emissivity values are on the order of 0.92. What would be the radiant
thermal flux per square meter from a brick wall at this temperature?
W=oT
W = 0.92 x 5.6697 x 10%(44+237)4
W = 527 w/n?
2 [Page
> Wien’s Displacement Law
* illustrates that the peak wavelength, Amax um) at which a surface radiates, is
easily determined by dividing a constant b (approximately 2897) by the absolute
temperature T (Kelvin) of the surface.
Anax = b/T
Where,
Anax is peak wavelength, pm
D is Wien’s displacement constant, b = 2897.
eax = 2897/T
= The spectral region in which the radiation is concentrated depends on
temperature, with comparatively mote radiation appearing ot SEA
* Example 1)
‘The peak emitting wavelength of a 300 °C (572 * F) blackbody is approximately:
hex = 2897/T
T geivin = T celsius + 273. 16
T kevin = 300 + 273.16 =573. 16 k
Anax = 2897/5783. 16
Anax = 5.05496 pm
23 [Page
4 Practical Infrared Measurements
> In practical measurement applications, the radiant energy leaves a target surface,
passes through some transmitting medium, usually an atmospheric path and reaches
a measuring instrument.
> as per the figure below, when making measurements or producing a thermogram,
three sets of characteristics must be considered:
a) characteristics of the target surface,
b) characteristics of the transmitting medium and
¢) characteristics of the measuring instrument.
Figure: Three sets of characteristics of the infrared measurement problem
The Total Infrared Measurement Situation
el
| sofa cf a
ecco Display
4 Characteristics of the Target Surface
“> Target surfaces are separated into three categories; blackbodies, graybodies and
nongraybodies
1. Blackbody, blackbody radiator
*_ Aperfect emitter; an object that absorbs all the radiant energy impinging on it at all
Wavelengths and reflects and transmits none,
* Asurtface with emissivity of unity (1.0) at all wavelengths. There is therefore no reflection
or transmission of the radiation.
2. Graybody ~ Graybody radiator
* Unlike blackbody radiators, graybody radiators never absorb all of the incident infrared
radiation, With a graybody radiator, some of the incident radiation is always reflected by
the surface and sometimes even transmitted (let through).
* Aradiating object whose emissivity is a constant value less thalllunity (110) over a specific
spectral range.
The emissivity of a graybody radiator is ahways independent of its temperature.
3. Nongraybody — Nongraybody radiator
Almost all naturally occurring objects are described as called Hal BEY) SCIEN
* Aradiating object that does not have a spectral radiation distribution similar to a
blackbody and can be partly transparent to infrared (transmits infrared energy at certain
wavelengths}.
= anemissivity varies not depend Shily With temperatire|Bubalso With spectral
wavelength,
* Glass and plastic films are examples of nongraybodies.
= Most metals are E6loredlFadiatOrs, which is why the emissivity of aluminum, for
‘example, increases when it is heated ( = 0.02 at 25 °C, © = 0.03 at 100 “C)!
“ Most radiation sources are not blackbodies. Some of the energy incident upon them
may be reflected or transmitted. The ratio of the radiant emittance W'of such a source
and the radiant emittance W of a blackbody at the same temperature is called the
emissivity € of the source:
e=W/W
> With this relation,
fferent types of radi
In the figure below “Spectral radiant emittance of three types of radiators” where
jon sources can be classified as indicated
a) The curve for the blackbody with e = Lis Plank's curve.
b) The curve for a graybody is proportional to Planck's curve for all wavelengths.
©) The spectral radiant emittance for a selective radiator varies not depend only
with temperature but also with wavelength.
Ww,
a
Blackbody (e = 1)
Selective Radiator (¢ = f(A,T))
Graybody (e < 1)
+ Example, Figure below shows the comparative spectral distribution of energy emitted
bya blackbody, a graybody and a nongraybody, all at the same temperature (300 K).
Figure Spectral distribution of a blackbody, graybody and nongraybody
Blackbody at 300 K
i
Graybody at 300 K
Nongraybody at 300 K
Target Relative Spectral Radiance
a
0
2
10
1 5 10 6 20
Wavelength (um)
4 Characteristics of the Transmitting Medium
* Because the infrared radiation from the target passes through some transmitting
medium on its way to the target, the transmission and emission characteristics of the
medium in the measurement path must be considered when making non-contact
thermal measurement.
+ No loss of energy or self-emission is encountered when measuring through a vacuum.
However, most measurements are made through air.
‘For short path length (a few meters, for example), most gases
cluding the
atmosphere) absorb and emit very little energy and can be ignored. However, when
highly accurate temperature measurements are required, the effects of atmospheric
absorption must be taken into account.
“As the path length increases to more than a few meters, or as the air becomes heavy
with water vapor, atmospheric absorption may become a significant factor. Therefore,
it is necessary to understand the infrared transmission characteristics of the
atmosphere.
26 Page
“As shown in Figure below, Atmospheric attenuation (white areas) with a chart of the
gases and water vapor causing most of it. The areas under the curve represent the
highest IR transmission.
> As the main part of the ‘window ‘spectrum, the atmosphere transmits infrared
energy very efficiently in the ESHA) spectral region.
> The infrared atmospheric ‘window’ spectrum that transmits infrared radiation
best s {HEBORSHLO UA region.
Transmittance ipercent)
eI
> water vapor (H20) will completely or partially absorb infrared electromagnetic energy
In the wave band of 6-8 um.
atmospheric
solar window window
absorption (%)
wavelength (um)
be uv —fovis}-— near 1p —f-— far 1R (longwave, thermal) —
27| Page
“ As shown in Figure below, Transmission, absorption and reflectance characteristics of
lass
> The spectral band in which glass transmits infrared radiation best is the BORO 310
Biiregion.
> Reflectance of infrared radiation by a glass surface is greatest in the 9O%0400Km
region.
Transmission of
Glass Envelopes
jonse
eflectance
1
ral Re:
and
3
ce
3
Absorption Reflectance of 1.3mm
Thick Glass Sample
feta
Relative S,
Percent Transmi
aan ee ee ea
A Waveléngth (um)
> Most significant is the fact that glass does not transmit infrared eneray at Om
where ambient (30°C, 86 °F) surfaces radiate their peak energy.
> When there is a solid material, such as a glass or quartz viewing port, between the
target and the instrument, the spectral characteristics of the solid media must be
known and considered.
> In practice, infrared thermal measurements of ambient targets can never be made
‘through glass. One practical approach to this problem is to eliminate the glass, or at
least a portion through which the instrument can be aimed at the target. If a window
must be present for personal safety, vacuum, or product safety, a material might be
substituted that transmits in the longer wavelengths.
28| Page
+ Figure below shows the spectral transmission characteristics of several infrared
transmitting materials
> many of which transmit energy pastaOumn.
> In addition to being used as transmitting windows, these materials are often used as
[SRESREEESUSIERIEH i infrared sensors and imagers.
> Of course, as targets become hotter, and the emitted energy shifts to the shorter
wavelengths, glass and quartz windows pose less of a problem and are even used as
elements and lenses in high temperature sensing instruments.
Figure Transmission curves of various infrared transmitting material
Germanium (ar-coated at 10 um) KRS-S
sugssszess
Percent Transmission
§
é
eS
Wavelength (um)
Zinc Selenide (ZnSe)
sugessssss
sugessaass
4
Percent Transmission
Percent Transmission
15 ry 1 . 6 ry
5 10 70
Wavelength (iim) Wavelength (usm)
Fused Quartz (SiO,) Barium Fluoride (BaF,)
susessasss
sugasssses
Percent Transmission
29| Page
You might also like
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Welding Engineering by Olga Gil - by WWW - LearnEngineering.inDocument114 pagesWelding Engineering by Olga Gil - by WWW - LearnEngineering.invignesh seenirajNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Ac7114 1 Rev H Audit Criteria For Nondestructive Testing Facility Penetrant Survey 1Document33 pagesAc7114 1 Rev H Audit Criteria For Nondestructive Testing Facility Penetrant Survey 1vignesh seenirajNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (346)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- E2297 22942 PDFDocument5 pagesE2297 22942 PDFvignesh seenirajNo ratings yet
- Strategic Management FrameworkDocument24 pagesStrategic Management Frameworkvignesh seenirajNo ratings yet
- Ut BasicDocument12 pagesUt Basicvignesh seenirajNo ratings yet
- T Trainin NG Cour Rse On Radiog Graphy Y Testi NG Lev VEL-2Document9 pagesT Trainin NG Cour Rse On Radiog Graphy Y Testi NG Lev VEL-2vignesh seenirajNo ratings yet
- Industrial Sickness in India: Causes and Consequences: Popular ArticleDocument3 pagesIndustrial Sickness in India: Causes and Consequences: Popular Articlevignesh seenirajNo ratings yet
- E) A'L, Wcci 4.N'H (Oi: McfirDocument2 pagesE) A'L, Wcci 4.N'H (Oi: Mcfirvignesh seenirajNo ratings yet
- Sandfits Foundries Private LimitedDocument7 pagesSandfits Foundries Private Limitedvignesh seenirajNo ratings yet
- SodaPDF Converted Skill Development FormDocument4 pagesSodaPDF Converted Skill Development Formvignesh seenirajNo ratings yet
- What Is ASTM E3022 18 and Why Do We Need ItDocument25 pagesWhat Is ASTM E3022 18 and Why Do We Need Itvignesh seenirajNo ratings yet
- A Reliability and Validity of An Instrument To Evaluate The School-Based Assessment System: A Pilot StudyDocument10 pagesA Reliability and Validity of An Instrument To Evaluate The School-Based Assessment System: A Pilot Studyvignesh seenirajNo ratings yet
- STAT15S - PSPP: Exercise Using PSPP To Explore Multiple Linear RegressionDocument5 pagesSTAT15S - PSPP: Exercise Using PSPP To Explore Multiple Linear Regressionvignesh seenirajNo ratings yet