Professional Documents
Culture Documents
Combination of RT-QPCR Testing and Clinical Features For Diagnosis of Covid-19 Facilitates Management of Sars-Cov-2 Outbreak
Uploaded by
Private HunterOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Combination of RT-QPCR Testing and Clinical Features For Diagnosis of Covid-19 Facilitates Management of Sars-Cov-2 Outbreak
Uploaded by
Private HunterCopyright:
Available Formats
Xuefeng Liu ORCID iD: 0000-0002-9922-9627
Combination of RT-qPCR Testing and Clinical Features For
Diagnosis of COVID-19 facilitates management of
SARS-CoV-2 Outbreak
Accepted Article
Yishan Wang1,* Hanyujie Kang1,*, Xuefeng Liu2, #, Zhaohui Tong1,#
1 Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory
Medicine, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, 100020,
China
2 Department of Pathology, Georgetown University Medical Center, Washington DC
20057
#
Correspondence to: Zhaohui Tong, Department of Respiratory and Critical Care
Medicine, Beijing Institute of Respiratory Medicine, Beijing Chao-Yang Hospital, Capital
Medical University, Beijing, 100020, China tongzhaohuicy@sina.com,
Xuefeng Liu, Department of Pathology, Georgetown University Medical Center,
Washington DC 20057 xuefeng.liu@georgetown.edu
Running title: Clinical Diagnosis of COVID-19
Keywords: Coronavirus, Virus classification, Respiratory tract, Pathogenesis, SARS
coronavirus, Virus classification
This article has been accepted for publication and undergone full peer review but
has not been through the copyediting, typesetting, pagination and proofreading
process, which may lead to differences between this version and the Version of
Record. Please cite this article as doi: 10.1002/jmv.25721.
This article is protected by copyright. All rights reserved.
In December 2019, a cluster of acute respiratory illness occurred in Wuhan,
Hubei Province, China. This disease is now officially known as 2019 novel
coronavirus disease (COVID-19) from WHO, novel coronavirus pneumonia
Accepted Article
(NCP) from Chinese Health Authorities, or SARS 2.0 from group
discussions, caused by SARS-CoV-2 or 2019 nCoV.1 Up to Feb 15, 2020,
66581 cases have been confirmed along with 8969 suspected cases in the
country and 37914 confirmed cases in Wuhan. Internationally, cases have
been reported in 24 countries and 5 continents. On February 12, a sudden
spike in new cases with COVID-19 (15152 new cases) was due to changed
diagnosis method according to the fifth edition of guidance,2 combination of
SARS-CoV-2 Nucleic Acid Test and clinical COVID-19 features.
Quantitative real-time RT-PCR (RT-qPCR) assay has routinely been
used for detection of causative viruses from respiratory secretions and final
pathogenic diagnostics of COVID-19. More than seven types of
SARS-CoV-2 nucleic acid test kit have been developed and approved
rapidly, while a large number of the “suspected” cases with typical clinical
COVID-19 features and identical specific CT images were not diagnosed.
Unfortunately, due to overwhelming situation in local hospitals, many
“suspected” cases and diagnosed cases cannot efficiently separated or
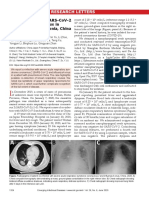
treated. Recently, one patient was not confirmed by RT-qPCR testing for
This article is protected by copyright. All rights reserved.
SARS-CoV-2 infection for the first three times within three weeks before
bronchoalveolar lavage fluid(BALF)was acquired, results from both
RT-qPCR and next-generation sequencing (NGS) testing were positive for
SRAS-CoV-2. These largely affected efficiency to control viral spreading
Accepted Article
and outbreak. Indeed, several factors have been proposed to explain the
inconsistency or the high false negative rate (FNR).3 For example, results
from RT-qPCR testing using primers in ORF1ab gene and N genes can be
affected by the variation of viral RNA sequences. In terms of natural history
of the disease and viral load in different anatomic sites of the patients,
sampling procedures largely contribute high FNR. By estimate, FNR from
one time testing was high as 30-50% in real COVID-19 cases.
It is urgent to rapidly optimize quality of testing kit and standard
operating procedure (SOP) for the best testing of SARS-CoV-2 infection.
Based on the sequence analysis of SARS-CoV-2 and ACE2 as a viral
receptor, we urgently recommend that samples from lower respiratory tract
of the patients, including sputum and BALF should be used for testing viral
infection although nasopharyngeal swab is more commonly used and
easier. Other factors or methods we should consider to further decrease
high FNR include sample reagents (for example, TRIZOL has been proved
for stability of RNA samples and can inactivate viruses), sample transport
This article is protected by copyright. All rights reserved.
condition, and laboratory practice standard. Lastly, developing
serum-based testing methods, for example, detection of
SARS-CoV-2-specific IgM from patients’ sera.
Accepted Article
Unlike SARS-CoV and MERS-CoV, SARS-CoV-2 is spreading faster,
initially COVID-19 may present without symptoms, or develop into fever,
coughing, shortness of breath, pain in the muscles and tiredness. As SARS
outbreak in 2003, some cases with COVID-19 may also develop into
pneumonia and acute respiratory distress syndrome (ARDS), which
contributed about 9% of death from the patients with severe conditions.
Previous studies have tested sensitivity and specificity for clinical diagnosis
of severe acute respiratory syndrome (SARS).3-4 Study showed a
sensitivity of 0.96 and a specificity of 0.96 for SRAS clinical diagnosis
based on SARS disease course.3 In the fifth edition of diagnosis and
treatment of COVID-19,5 clinical diagnosis was proposed for the first time.
This method has been used to define cases in Hubei province which have
epidemiology history, clinical features (①fever and/or respiratory
symptoms;②early onset of normal/decreased white blood cell count or
decreased lymphocyte count) along with sign of pneumonia on chest CT
scan/X-ray. As described in a latest preprint article with a large number of
cases (1099 cases) with COVID-19, the most common clinical and
This article is protected by copyright. All rights reserved.
radiographic features were summarized from a large number of cases with
COVID-19.6 Fever and cough were the most common symptoms,
lymphopenia was very common on admission, severe cases appeared to
have more prominent laboratory abnormalities. The most common patterns
Accepted Article
(76.4%) on chest computed tomography (CT) were ground-glass opacity
and bilateral patchy shadowing. The study also disclosed a case with
24-day incubation period for the first time, which was claimed as a unique
case by one of the co-author.6 These CT patterns have been found in many
“suspected” cases with negative testing of SARS-CoV-2 viral RNA in
hospitals in Wuhan, China.
As we learn more about SARS-CoV-2 and COVID-19, we began to
realize the deficiency in the diagnosis based on sole detection of viral
nucleic acid, diagnosis with combination of RT-qPCT or NGS testing and
clinical criteria may be more important for management of the current
outbreak in China, especially in Wuhan. Since Chinese government
launched an urgent policy for all diagnosed patients with COVID-19, there
is no cost for all treatments from the COVID-19. This change would
significantly facilitate sufficient management of SARS-CoV-2 outbreak in
Wuhan, especially increase patient compliance for quarantine and
treatments.
This article is protected by copyright. All rights reserved.
Author contributions: YW and HK contributed equally to this work.
Conception or design of the work: YW, HK, ZT; Data collection: YW, HK;
Drafting the article: YW, HK; Critical revision of the article: ZT, XL; Final
approval: YW, HK, XL, ZT
Accepted Article
CONFLICT OF INTEREST: Authors declare that they have no conflict of
interest.
Reference
1. Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory
syndrome-related coronavirus: The species and its viruses – a statement of
the Coronavirus Study Group.
doi: https://doi.org/10.1101/2020.02.07.937862
2. Xi M, Wei Q, Qihua F, et al. Understanding the Influence Factors in Viral
Nucleic Acid Test of 2019 novel Coronavirus (2019-nCoV). Chin J Lab Med
2020; 43(00): E002-E002.
3. Rainer TH, Chan PK, Ip M, et al. The spectrum of severe acute
respiratory syndrome-associated coronavirus infection. Ann Intern Med
2004; 140(8):614-619.
4. Ho PL, Chau PH, Yip PS, et al. A prediction rule for clinical diagnosis of
severe acute respiratory syndrome. Eur Respir J 2005; 26: 474–479.
This article is protected by copyright. All rights reserved.
5. diagnosis and treatment of novel coronavirus pneumonia (5th edition).
6. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel
coronavirus infection in China. medRxiv 2020;
Accepted Article
doi.org/10.1101/2020.02.06.20020974
This article is protected by copyright. All rights reserved.
You might also like
- Pato Job 2Document21 pagesPato Job 2David Njuguna100% (1)
- Answering Crucial Questions About Sars-CoV-2Document57 pagesAnswering Crucial Questions About Sars-CoV-2Sarah WestallNo ratings yet
- Luc Montagnier - Molecular Genetics of SARS-COV-2 - 678-Article Text-4089-3-10-20200806Document47 pagesLuc Montagnier - Molecular Genetics of SARS-COV-2 - 678-Article Text-4089-3-10-20200806Sandra100% (1)
- (New Paradigms in Healthcare) Maria Giulia Marini, Jonathan McFarland - Health Humanities For Quality of Care in Times of COVID - 19-Springer (2022)Document175 pages(New Paradigms in Healthcare) Maria Giulia Marini, Jonathan McFarland - Health Humanities For Quality of Care in Times of COVID - 19-Springer (2022)Sofronie MadalinNo ratings yet
- 2022-10-28 - Petitioner Requests Respondent Re-Assess Circumstance of 12 September 2022 Hospital OrderDocument15 pages2022-10-28 - Petitioner Requests Respondent Re-Assess Circumstance of 12 September 2022 Hospital OrderCanadian Society for the Advancement of Science in Public PolicyNo ratings yet
- (14374331 - Clinical Chemistry and Laboratory Medicine (CCLM) ) Prominent Changes in Blood Coagulation of Patients With SARS-CoV-2 InfectionDocument5 pages(14374331 - Clinical Chemistry and Laboratory Medicine (CCLM) ) Prominent Changes in Blood Coagulation of Patients With SARS-CoV-2 InfectionMahtosurup GodavarthyNo ratings yet
- Clinical Characteristics of Severe Acute Respiratory Syndrome Coronavirus 2 Reactivation Guangming Ye Download 2024 Full ChapterDocument23 pagesClinical Characteristics of Severe Acute Respiratory Syndrome Coronavirus 2 Reactivation Guangming Ye Download 2024 Full Chaptervito.lyons426100% (11)
- Covide 19Document12 pagesCovide 19Hamza MehalliNo ratings yet
- Covid UpdateDocument25 pagesCovid UpdateAndreea PostolacheNo ratings yet
- Recurrence of Positive Sars Cov 2 Rna in Covid 19 A Case Report Dabiao Chen Download 2024 Full ChapterDocument25 pagesRecurrence of Positive Sars Cov 2 Rna in Covid 19 A Case Report Dabiao Chen Download 2024 Full Chapterrichard.byrd946100% (10)
- Diagnostic Aspect of COVIDDocument8 pagesDiagnostic Aspect of COVIDYanMardianNo ratings yet
- Journal Pone 0262820Document11 pagesJournal Pone 0262820bioNo ratings yet
- 2019 Novel Coronavirus Disease (COVID-19) in Taiwan: Reports of Two Cases From Wuhan, ChinaDocument4 pages2019 Novel Coronavirus Disease (COVID-19) in Taiwan: Reports of Two Cases From Wuhan, ChinaClaav HugNo ratings yet
- Httpsaulavirtual - Ucentral.clpluginfile - Php418334mod resourcecontent2Grupo20V20Seminario - Pdfredirect 1Document11 pagesHttpsaulavirtual - Ucentral.clpluginfile - Php418334mod resourcecontent2Grupo20V20Seminario - Pdfredirect 1Belen SozaNo ratings yet
- 2021 Article 5829Document11 pages2021 Article 5829Demian HerreraNo ratings yet
- Insight Into 2019 Novel Coronavirus An Updated Intrim Review and Lessons From Sars Cov and Mers Cov Mingxuan Xie Download 2024 Full ChapterDocument45 pagesInsight Into 2019 Novel Coronavirus An Updated Intrim Review and Lessons From Sars Cov and Mers Cov Mingxuan Xie Download 2024 Full Chaptertom.beckett574100% (10)
- Prominent Changes in Blood Coagulation of Patients With Sars-Cov-2 InfectionDocument10 pagesProminent Changes in Blood Coagulation of Patients With Sars-Cov-2 InfectionMuhammad Rizky CaniagoNo ratings yet
- Journal Pre-Proof: Clinical ImmunologyDocument32 pagesJournal Pre-Proof: Clinical ImmunologyMiguel AngelNo ratings yet
- Real-Time RT-PCR For COVID-19 Diagnosis: Challenges and ProspectsDocument3 pagesReal-Time RT-PCR For COVID-19 Diagnosis: Challenges and ProspectsAnthony StackNo ratings yet
- A Case Series Describing The Epidemiology and Clinical Characteristics of COVID-19 Infection in Jilin ProvinceDocument4 pagesA Case Series Describing The Epidemiology and Clinical Characteristics of COVID-19 Infection in Jilin Provinceabeer alrofaeyNo ratings yet
- Antibody Responses To Sars-Cov-2 in Patients of Novel Coronavirus Disease 2019Document22 pagesAntibody Responses To Sars-Cov-2 in Patients of Novel Coronavirus Disease 2019cristinaNo ratings yet
- Laboratory Testing of Sars-Cov, Mers-Cov, and Sars-Cov-2 (2019-Ncov) : Current Status, Challenges, and CountermeasuresDocument14 pagesLaboratory Testing of Sars-Cov, Mers-Cov, and Sars-Cov-2 (2019-Ncov) : Current Status, Challenges, and CountermeasuresDanteraiantNo ratings yet
- BiomedPap - Bio 202003 0001Document9 pagesBiomedPap - Bio 202003 0001Edison Chiriboga OrtegaNo ratings yet
- Novel Dual Multiplex Real Time RT PCR Assays For The Rapid Detection of SARS CoV 2 Influenza A B and Respiratory Syncytial Virus Using The BD MAXDocument7 pagesNovel Dual Multiplex Real Time RT PCR Assays For The Rapid Detection of SARS CoV 2 Influenza A B and Respiratory Syncytial Virus Using The BD MAXHasna Mirda AmazanNo ratings yet
- Insight Into 2019 Novel Coronavirus-An Updated Intrim Review and Lessonsfrom SARS-CoV and MERS-CoVDocument22 pagesInsight Into 2019 Novel Coronavirus-An Updated Intrim Review and Lessonsfrom SARS-CoV and MERS-CoVronahaniifah11No ratings yet
- MainDocument7 pagesMainM ANo ratings yet
- 10.1515 - DX 2020 0091Document3 pages10.1515 - DX 2020 0091trisna amijayaNo ratings yet
- COVID-19 Screening Prognosis and Severity Assessment With Biomarkers HORIBA MedicalDocument14 pagesCOVID-19 Screening Prognosis and Severity Assessment With Biomarkers HORIBA MedicalIrkania PasangkaNo ratings yet
- COVID-19 Autopsies, Oklahoma, USADocument9 pagesCOVID-19 Autopsies, Oklahoma, USARhagaraRhaNo ratings yet
- Artikel Covid-19Document9 pagesArtikel Covid-19nhshivani532000No ratings yet
- Articulo Por TraducirDocument4 pagesArticulo Por TraducirAntonio Zumaque CarrascalNo ratings yet
- Asymptomatic Carrier State Acute Respiratory Disease and Pneumonia Due To Severe Acute Respiratory Syndrome Coronavirus 2 Sars Cov 2 Facts and Myths Chih Cheng Lai Download 2024 Full ChapterDocument32 pagesAsymptomatic Carrier State Acute Respiratory Disease and Pneumonia Due To Severe Acute Respiratory Syndrome Coronavirus 2 Sars Cov 2 Facts and Myths Chih Cheng Lai Download 2024 Full Chaptertyrone.cruz893100% (11)
- Evaluation of An Antigen Detection Rapid Diagnostic Test For Detection of Sars-Cov-2 in Clinical SamplesDocument9 pagesEvaluation of An Antigen Detection Rapid Diagnostic Test For Detection of Sars-Cov-2 in Clinical Samplesjytj tjrj64fdNo ratings yet
- International Journal of Infectious DiseasesDocument3 pagesInternational Journal of Infectious DiseasesDetti FahmiasyariNo ratings yet
- Cid RT PCR Positive Over TimeDocument12 pagesCid RT PCR Positive Over TimePatrick CoNo ratings yet
- Dynamics and Correlation Among Viral Positivity, Seroconversion, and Disease Severity in COVID-19Document15 pagesDynamics and Correlation Among Viral Positivity, Seroconversion, and Disease Severity in COVID-19yanuarizkaNo ratings yet
- Research Letters: Co-Infection With Sars-Cov-2 and Influenza A Virus in Patient With Pneumonia, ChinaDocument3 pagesResearch Letters: Co-Infection With Sars-Cov-2 and Influenza A Virus in Patient With Pneumonia, Chinajitesh141No ratings yet
- Ijs DR 2212055Document4 pagesIjs DR 2212055Nicolas CastilloNo ratings yet
- Réponse Immun Covid19Document8 pagesRéponse Immun Covid19Tidiane SibyNo ratings yet
- The Hemocyte Counts As A Potential Biomarker For Predicting Disease Progression in COVID-19: A Retrospective StudyDocument10 pagesThe Hemocyte Counts As A Potential Biomarker For Predicting Disease Progression in COVID-19: A Retrospective StudygiovannaNo ratings yet
- The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 PneumoniaDocument5 pagesThe Clinical and Chest CT Features Associated With Severe and Critical COVID-19 PneumoniaNadanNadhifahNo ratings yet
- COVID-19 Autopsies - Aqaa062Document9 pagesCOVID-19 Autopsies - Aqaa062Pramod BiradarNo ratings yet
- Case Report - Walking Pneumonia in Novel Coronavirus Disease Covid19Document3 pagesCase Report - Walking Pneumonia in Novel Coronavirus Disease Covid19Walter Pinto TenecelaNo ratings yet
- Medical Virology Jan 2021Document9 pagesMedical Virology Jan 2021cdsaludNo ratings yet
- Correlation Between Ground-Glass Opacity On Pulmonary CT and The Levels of Inflammatory CytokinesDocument7 pagesCorrelation Between Ground-Glass Opacity On Pulmonary CT and The Levels of Inflammatory CytokinesEarlya RifaniNo ratings yet
- USS 2020-I. - Autop - COVID19Document9 pagesUSS 2020-I. - Autop - COVID19Jerry Carhuatanta SaavedraNo ratings yet
- Epidemiologic and Clinical Characteristics of 91 Hospitalized Patients With COVID-19 in Zhejiang, China: A Retrospective, Multi-Centre Case SeriesDocument9 pagesEpidemiologic and Clinical Characteristics of 91 Hospitalized Patients With COVID-19 in Zhejiang, China: A Retrospective, Multi-Centre Case SeriesVeloz RedNo ratings yet
- 001 Profiling Early Humoral Response To COVID-19 (IDSA 20) READDocument8 pages001 Profiling Early Humoral Response To COVID-19 (IDSA 20) READagboxNo ratings yet
- Bostan-2020-Targets and Assay Types For COVIDDocument15 pagesBostan-2020-Targets and Assay Types For COVIDNick FloresNo ratings yet
- COVID19 PCP AIDS 2020 Case ReportDocument4 pagesCOVID19 PCP AIDS 2020 Case ReportmingNo ratings yet
- Higher Level of Neutrophil-to-Lymphocyte Is Associated With Severe COVID-19Document12 pagesHigher Level of Neutrophil-to-Lymphocyte Is Associated With Severe COVID-19Emier HidayatNo ratings yet
- 1 s2.0 S0272638620306120 ManinDocument10 pages1 s2.0 S0272638620306120 ManinrezaNo ratings yet
- Journal of Clinical Microbiology-2020-Chan-JCM.00310-20.full PDFDocument33 pagesJournal of Clinical Microbiology-2020-Chan-JCM.00310-20.full PDFdavid zhangNo ratings yet
- InternacionalDocument9 pagesInternacionalbryan ronaldNo ratings yet
- Immune Responses in COVID-19 and Potential Vaccines: Lessons Learned From SARS and MERS EpidemicDocument9 pagesImmune Responses in COVID-19 and Potential Vaccines: Lessons Learned From SARS and MERS EpidemicCucumber IsHealthy96No ratings yet
- Journal Pre-Proof: Journal of Clinical VirologyDocument12 pagesJournal Pre-Proof: Journal of Clinical VirologymiNo ratings yet
- Pruebas Diagnosticas Covid-19Document7 pagesPruebas Diagnosticas Covid-19JADIT YASIRA MENDOZA HERNANDEZNo ratings yet
- Dysregulation of Immune Response in Patients With COVID-19 in Wuhan, ChinaDocument24 pagesDysregulation of Immune Response in Patients With COVID-19 in Wuhan, ChinaChavdarNo ratings yet
- Coronavirus3 PDFDocument28 pagesCoronavirus3 PDFWilmar David Medina MadridNo ratings yet
- Immune Responses in COVID-19 and Potential Vaccines: Lessons Learned From SARS and MERS EpidemicDocument9 pagesImmune Responses in COVID-19 and Potential Vaccines: Lessons Learned From SARS and MERS EpidemicJC XDNo ratings yet
- Presence of Mismatches Between Diagnostic PCR Assays and Coronavirus Sars-Cov-2 GenomeDocument17 pagesPresence of Mismatches Between Diagnostic PCR Assays and Coronavirus Sars-Cov-2 GenomeYulaimy BatistaNo ratings yet
- Favipiravir Control StudyDocument7 pagesFavipiravir Control Studyadel ehabNo ratings yet
- COVID-19 and Multi-Organ ResponseDocument31 pagesCOVID-19 and Multi-Organ ResponseArbaz SultanNo ratings yet
- Remdesivir For Severe Acute Respiratory Syndrome Coronavirus 2 Causing Covid 19 An Evaluation of The Evidence Yu Chen Cao All ChapterDocument53 pagesRemdesivir For Severe Acute Respiratory Syndrome Coronavirus 2 Causing Covid 19 An Evaluation of The Evidence Yu Chen Cao All Chapterdora.morales550100% (15)
- D Llysine Acetylsalicylate Glycine Impairs Coronavirus Replication Jaa 1000151Document9 pagesD Llysine Acetylsalicylate Glycine Impairs Coronavirus Replication Jaa 1000151misancheNo ratings yet
- Department of Genetics: Covid-19 RT PCRDocument1 pageDepartment of Genetics: Covid-19 RT PCRsoniyaNo ratings yet
- Department of Genetics: Covid-19 RT PCRDocument1 pageDepartment of Genetics: Covid-19 RT PCRSahil AnsariNo ratings yet
- Topic - 5 - Mers - Cov - (7) FINALDocument12 pagesTopic - 5 - Mers - Cov - (7) FINALApril FlorendoNo ratings yet
- Tabuk City National High SchoolDocument14 pagesTabuk City National High SchoolMark NerbNo ratings yet
- Nano Today: Marcel Alexander Heinrich, Byron Martina, Jai PrakashDocument21 pagesNano Today: Marcel Alexander Heinrich, Byron Martina, Jai Prakashvishal makadiaNo ratings yet
- Li-Meng Yan Report 2Document35 pagesLi-Meng Yan Report 2imnotbncre8iveNo ratings yet
- Jurnal BHS Inggris FixDocument10 pagesJurnal BHS Inggris FixadilaNo ratings yet
- Long COVID or Post-Acute Sequelae of COVID-19 (PASC) - An Overview of Biological Factors That May Contribute To Persistent SymptomsDocument24 pagesLong COVID or Post-Acute Sequelae of COVID-19 (PASC) - An Overview of Biological Factors That May Contribute To Persistent SymptomsRicardo Pariona LlanosNo ratings yet
- EMI Conspiracy ZlshiDocument10 pagesEMI Conspiracy ZlshiOstflopNo ratings yet
- Covid 19Document22 pagesCovid 19imad khanNo ratings yet
- Structural Analysis of Sequence Data in Virology An Elementary Approach Using Sars-Cov-2 As An ExampleDocument35 pagesStructural Analysis of Sequence Data in Virology An Elementary Approach Using Sars-Cov-2 As An ExampleAdrian BoboceaNo ratings yet
- Module 7 Communication For Academic PurposesDocument50 pagesModule 7 Communication For Academic PurposesJoksian TrapelaNo ratings yet
- MAPEH 10 - Q1 - W3 - Mod3Document37 pagesMAPEH 10 - Q1 - W3 - Mod3Jeraldine MendozaNo ratings yet
- Corona Virus Variants: Sars-Cov - Mers-Cov - CovidDocument25 pagesCorona Virus Variants: Sars-Cov - Mers-Cov - CovidJenny Agustin FabrosNo ratings yet
- MT1 PMLS Lec L9Document7 pagesMT1 PMLS Lec L9SEBASTIEN ANDREI BUENAFENo ratings yet
- Compiled ProjectDocument74 pagesCompiled ProjectMISHEAL CHIEMELANo ratings yet
- Wetransfer - ProjectDocument40 pagesWetransfer - ProjectĐinh ThắngNo ratings yet
- SeqdumpDocument7 pagesSeqdumpAnayantzin AyalaNo ratings yet
- Corona ProfilDocument19 pagesCorona ProfilVincentius Michael WilliantoNo ratings yet
- Meteorological Parameters and Air PollutantsDocument8 pagesMeteorological Parameters and Air PollutantssyamsuddinNo ratings yet
- Group 3 - OPORD Bakunahan Sa Norte - Final Output-15Jan22Document92 pagesGroup 3 - OPORD Bakunahan Sa Norte - Final Output-15Jan22Marlon MejiaNo ratings yet
- Dushyant Kumar RTPCR Apollo 01022022Document2 pagesDushyant Kumar RTPCR Apollo 01022022tabrez ahmadNo ratings yet
- Molecules 25 05091 v2Document25 pagesMolecules 25 05091 v2MishtiNo ratings yet
- The Sustained Impact of Tension Induced by Covid On The Food Service Industry Employees Gilles DraftDocument79 pagesThe Sustained Impact of Tension Induced by Covid On The Food Service Industry Employees Gilles DraftIbeanu ChimaNo ratings yet