Professional Documents
Culture Documents
Covid Results - Destiny Carter - Cor-21-223596
Uploaded by
Regina Halmon0 ratings0% found this document useful (0 votes)
21 views1 pageOriginal Title
Covid Results - Destiny Carter_cor-21-223596
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
21 views1 pageCovid Results - Destiny Carter - Cor-21-223596
Uploaded by
Regina HalmonCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
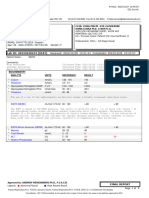
3495 Hacks Cross Road, Memphis, TN 38125
Tel (901) 526-7444 Fax (901) 526-0791
Toll Free: (888) 2GI-PATH
Richard S Kinsey, M.D., Laboratory Director
CLIA # 44D0915029
Patient: CARTER, DESTINY Accession Number: COR-21-223596
Address: 1563 Barton St Office Visit Date: 8/20/2021 1:29 PM
MEMPHIS, TN 38106 Specimen Received: 8/20/2021
Gender: F DOB: 5/2/2009 Age: 12 MR/Chart: 19346593/
Physician: Jackie Makapugay, MD Referring
Client: PHC DRIVE THRU TESTING Physician:
SOURCE OF SPECIMEN: NASAL SWAB
CLINICAL HISTORY: Headache; Nausea
SARS-CoV-2 RESULT:
Pathogen Name Result
SARS-CoV-2 RNA NEGATIVE
pan-Sarbecovirus including SARS-CoV-2 NEGATIVE
COVID-19 assay is a real-time RT-PCR test intended for the presumptive qualitative detection of nucleic acid from SARS-CoV-2 in
nasopharyngeal swab, nasopharyngeal aspirate, and bronchoalveolar lavage (BAL) specimens from individuals meeting CDC COVID-
19 clinical criteria (e.g., clinical signs and symptoms associated with SARS-Cov-2 infection) in conjunction with CDC COVID-19
epidemiological criteria (e.g., history of residence in or travel to a geographic region with active SARS-CoV-2 transmission at the time of
travel, or other epidemiologic criteria for which SARS-CoV-2 testing may be indicated.
Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for patient management decisions.
Negative results must be combined with clinical observations, patient history, and epidemiological information.
This test was developed by Roche on COBAS® 6800/8800 and its performance characteristics verified by Poplar Healthcare. Cobas®
SARS-CoV-2 test is FDA approved under Emergency Use Authorization (EUA). Poplar Healthcare is regulated under CLIA as qualified
to perform high-complexity testing.
Anami Patel, Ph.D. MB (ASCP) CM DLM CM
Chief Science Officer
Electronically signed Aug 20, 2021
Date Reported: Aug 20, 2021
CPT Code: U0003
ICD Code:
You might also like
- Result LetterDocument2 pagesResult Letterbilalazam31100% (1)
- Nutrition Through-Out Lifespan (Infancy)Document37 pagesNutrition Through-Out Lifespan (Infancy)Vincent Maralit MaterialNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- CGH202011008542 Lab-2020-0351447 Laboratory Covid-Pcr-Test PDFDocument2 pagesCGH202011008542 Lab-2020-0351447 Laboratory Covid-Pcr-Test PDFMae SampangNo ratings yet
- WSET L1 2 Wines SpecificationDocument46 pagesWSET L1 2 Wines Specificationcipollina666No ratings yet
- Genome Sciences Building SID: CTTP-005-8551 Final - Approved 01/14/2021 5:10PM Collected: 01/13/2021 1:39PMDocument1 pageGenome Sciences Building SID: CTTP-005-8551 Final - Approved 01/14/2021 5:10PM Collected: 01/13/2021 1:39PMJames C.100% (1)
- Method Statement - Foul Drainage DiversionDocument4 pagesMethod Statement - Foul Drainage DiversionBNo ratings yet
- 0 - Tiara WilliamsDocument2 pages0 - Tiara WilliamsTiara WilliamsNo ratings yet
- WebrepDocument1 pageWebrepMayur ThebossNo ratings yet
- Rawat, Baldev Singh: Negative CRSP Covid19 - Diagnostic Source: AN SwabDocument1 pageRawat, Baldev Singh: Negative CRSP Covid19 - Diagnostic Source: AN SwabBaldev RawatNo ratings yet
- Laboratory Report: Patient: Ordering PhysicianDocument1 pageLaboratory Report: Patient: Ordering PhysicianJake MorganNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- COVID-19 Report - Mr. Ashish Gudka, Executive Assistant To MD & Group CEODocument2 pagesCOVID-19 Report - Mr. Ashish Gudka, Executive Assistant To MD & Group CEODeepak UpadhayayNo ratings yet
- Covidl 9 Test - Google Drive: St. Luke'sDocument2 pagesCovidl 9 Test - Google Drive: St. Luke'sAya BeeNo ratings yet
- Report 4Document2 pagesReport 4Tahira HashmiNo ratings yet
- Test Description Results Units Reference Range Abnormal Lab: Moutou, MathieuDocument2 pagesTest Description Results Units Reference Range Abnormal Lab: Moutou, MathieuMathieu François MoutouNo ratings yet
- Ali, Zahir: SWAB + COVID19 (Package) (Final Report)Document2 pagesAli, Zahir: SWAB + COVID19 (Package) (Final Report)Muzyan MominNo ratings yet
- WebrepDocument1 pageWebrepArana ImportacionesNo ratings yet
- Covid ResultDocument1 pageCovid ResultMiles LabadoNo ratings yet
- Lab ID: 20233620234 (STAT) Received: 2023/12/28 13:51:41 Completed: 2023/12/28 15:23:57Document5 pagesLab ID: 20233620234 (STAT) Received: 2023/12/28 13:51:41 Completed: 2023/12/28 15:23:57Liu HànNo ratings yet
- Abreu, CarlosDocument1 pageAbreu, CarlosCarlos AbreuNo ratings yet
- Covid-19 RT-PCR: Test Results PanelDocument1 pageCovid-19 RT-PCR: Test Results PanelPatricia Cottle-SalyerNo ratings yet
- Test Normal Abnormal Range Units: Page 1 of 1Document1 pageTest Normal Abnormal Range Units: Page 1 of 1HERNÀN CARABAÑO G.No ratings yet
- COVID-19 ReportDocument2 pagesCOVID-19 Reportsk9308346360No ratings yet
- Department of Genetics: Covid-19 RT PCRDocument1 pageDepartment of Genetics: Covid-19 RT PCRᴍᴏʜᴀᴍᴍᴇᴅ ʙɪʟᴀʟNo ratings yet
- Final Report 63519 999999999999Document1 pageFinal Report 63519 999999999999Rahim AlkabiNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not Detected (-)Document1 pageSars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not Detected (-)Richmond SantiagoNo ratings yet
- Philippine Red Cross Molecular Laboratory Result Form: Date: NameDocument1 pagePhilippine Red Cross Molecular Laboratory Result Form: Date: NameJohn De VillaNo ratings yet
- Test Name Result: Department of PathologyDocument2 pagesTest Name Result: Department of PathologyWil LanecraNo ratings yet
- COVID-19 Test Result Summary: NegativeDocument2 pagesCOVID-19 Test Result Summary: NegativeChristopher GodinezNo ratings yet
- TMCSL 20230112 0008Document1 pageTMCSL 20230112 0008Jared OcampoNo ratings yet
- Department of Genetics: Covid-19 RT PCRDocument1 pageDepartment of Genetics: Covid-19 RT PCRChirayil VarugheseNo ratings yet
- Covid-19 Qualitative PCR Not Detected Target Gene CT Value: D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703Document5 pagesCovid-19 Qualitative PCR Not Detected Target Gene CT Value: D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703Kirti SuryawanshiNo ratings yet
- r146105526 Sofia Caceres CUR146105526Document1 pager146105526 Sofia Caceres CUR146105526Sofía Beatriz Cáceres RamosNo ratings yet
- Madhan - 642161200148401 2Document2 pagesMadhan - 642161200148401 2madhanNo ratings yet
- Covid TestsDocument2 pagesCovid TestsMC KelNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19) Viral Rna Detected (+)Document1 pageSars-Cov-2 (Causative Agent of Covid-19) Viral Rna Detected (+)Richmond SantiagoNo ratings yet
- ReportDocument1 pageReportShawn JamesNo ratings yet
- Bashayreh-Bilal-01-02-2022 17 - 28PM 3Document1 pageBashayreh-Bilal-01-02-2022 17 - 28PM 3bilal bashayrehNo ratings yet
- Sars-Cov-2 (Covid-19) : Patient Specimen PhysicianDocument1 pageSars-Cov-2 (Covid-19) : Patient Specimen PhysicianBrayan AtiroNo ratings yet
- Department of Genetics: Covid-19 RT PCRDocument1 pageDepartment of Genetics: Covid-19 RT PCRliby chackoNo ratings yet
- Department of Genetics: Covid-19 RT PCRDocument1 pageDepartment of Genetics: Covid-19 RT PCRAswathy LNo ratings yet
- CGH202012022479 Lab-2020-0411918 Laboratory Covid-Pcr-TestDocument2 pagesCGH202012022479 Lab-2020-0411918 Laboratory Covid-Pcr-TestJosa Camille BungayNo ratings yet
- Myquest™ : Sars Cov 2 Rna (Covid 19), Qualitative NaatDocument2 pagesMyquest™ : Sars Cov 2 Rna (Covid 19), Qualitative NaatByron ViscarraNo ratings yet
- QCMDL 21 57987 Beltran Karen Villavicensio 1Document1 pageQCMDL 21 57987 Beltran Karen Villavicensio 1lemuel clausNo ratings yet
- LabResultTempPDF CJ0304865Document2 pagesLabResultTempPDF CJ0304865Jahred EstebanNo ratings yet
- Sachin KharatDocument3 pagesSachin KharatHarish GundaNo ratings yet
- Prophasedx Laboratory Phone: (855) 982-1100Document2 pagesProphasedx Laboratory Phone: (855) 982-1100ommanon15 aNo ratings yet
- QCMDL 21 51393 Relata Leonardo NacionalDocument1 pageQCMDL 21 51393 Relata Leonardo NacionalAngel DetablanNo ratings yet
- Manoriã - A, ElmaDocument1 pageManoriã - A, ElmaElmaNo ratings yet
- Clinical Lab Report: Test Result Flags Ref. Range UnitsDocument1 pageClinical Lab Report: Test Result Flags Ref. Range UnitsMichaela HessonNo ratings yet
- Covid-19 RTPCR (Sars Cov-2) Throat/Nasal Swab-Haryana : Laboratory Investigation ReportDocument1 pageCovid-19 RTPCR (Sars Cov-2) Throat/Nasal Swab-Haryana : Laboratory Investigation ReportkanavNo ratings yet
- Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument2 pagesSars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRBATARNo ratings yet
- D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: ThyrocareDocument3 pagesD-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: ThyrocareAmit RastogiNo ratings yet
- Floor, Vrindavan CHS, Shastri: Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument2 pagesFloor, Vrindavan CHS, Shastri: Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRHimanshu OzaNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- D-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Processed At: ThyrocareDocument3 pagesD-37/1, TTC MIDC, Turbhe, Navi Mumbai-400 703: Processed At: ThyrocareSahil VaishyaNo ratings yet
- April Jane: Cellular Immunology and ImmunogeneticsDocument2 pagesApril Jane: Cellular Immunology and ImmunogeneticsAya BeeNo ratings yet
- Take Care Sa GensanDocument1 pageTake Care Sa GensanAya BeeNo ratings yet
- DescargaDocument1 pageDescargaJacinto RoblesNo ratings yet
- Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument3 pagesSars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRVella YoutuberNo ratings yet
- Minimal Residual Disease Testing: Current Innovations and Future DirectionsFrom EverandMinimal Residual Disease Testing: Current Innovations and Future DirectionsTodd E. DruleyNo ratings yet
- Proteomics Today: Protein Assessment and Biomarkers Using Mass Spectrometry, 2D Electrophoresis,and Microarray TechnologyFrom EverandProteomics Today: Protein Assessment and Biomarkers Using Mass Spectrometry, 2D Electrophoresis,and Microarray TechnologyNo ratings yet
- SDD 048 ML 12xx DocumentDocument12 pagesSDD 048 ML 12xx DocumentRomiNo ratings yet
- How To Determine Fire Flow (IFC Method)Document6 pagesHow To Determine Fire Flow (IFC Method)Dani HambalinaNo ratings yet
- Trauma Informed Care Information From Allison Sampson Jackson PDFDocument14 pagesTrauma Informed Care Information From Allison Sampson Jackson PDFMirjana StevanovicNo ratings yet
- Material Considerations Irradiation Processing - SOTERADocument8 pagesMaterial Considerations Irradiation Processing - SOTERAeyalzuckermanNo ratings yet
- COMEDK UGET-2011 Medical Rank ListDocument147 pagesCOMEDK UGET-2011 Medical Rank ListiamvarkeyNo ratings yet
- Brunei 2Document16 pagesBrunei 2Eva PurnamasariNo ratings yet
- ĐỀ 9Document4 pagesĐỀ 9Hau NgoNo ratings yet
- Perceptions of Students RegardDocument6 pagesPerceptions of Students RegardLina Mahayaty SembiringNo ratings yet
- Esec 2100 XP Esec 2100 XP: Plus PlusDocument2 pagesEsec 2100 XP Esec 2100 XP: Plus PlusЧарли FrogNo ratings yet
- Anabolic PDFDocument21 pagesAnabolic PDFNe MiNo ratings yet
- Don Honorio Ventura Technological State University Bacolor, PampangaDocument10 pagesDon Honorio Ventura Technological State University Bacolor, PampangaAnonymous Xwd7uWe0YUNo ratings yet
- Athletic Code of Conduct REVISED 020717Document6 pagesAthletic Code of Conduct REVISED 020717NewsChannel 9No ratings yet
- Water ProofingDocument5 pagesWater ProofingMalith De SilvaNo ratings yet
- Vaccination List: Belyaletdinov, Ravil AbdulverovichDocument5 pagesVaccination List: Belyaletdinov, Ravil AbdulverovichCarlos MoriNo ratings yet
- Extended Zimbabwe National AIDS Strategic Plan 3Document80 pagesExtended Zimbabwe National AIDS Strategic Plan 3Mxolisi Ncube0% (1)
- Unit I Health Literacy For StudentsDocument11 pagesUnit I Health Literacy For StudentsNilam Putri Defa100% (1)
- MDR - Conformity AssessmentDocument8 pagesMDR - Conformity AssessmentNathan LabordeNo ratings yet
- SHDH2040 Lecture 5Document83 pagesSHDH2040 Lecture 5123 HahahaNo ratings yet
- FR QuestionsDocument6 pagesFR QuestionsAvisek MohantyNo ratings yet
- Pregnancy in Dental TreatmentDocument62 pagesPregnancy in Dental TreatmentChinar HawramyNo ratings yet
- Disersa GuatemalasurDocument340 pagesDisersa GuatemalasurGerson David Ortíz MoralesNo ratings yet
- 8662Document8 pages86628662No ratings yet
- Doc22 PDFDocument78 pagesDoc22 PDFAmelia BrownNo ratings yet
- Science Investigatory ProjectDocument17 pagesScience Investigatory ProjectVhon AndreaNo ratings yet
- Aipmt-Neet Cutoff 2012Document178 pagesAipmt-Neet Cutoff 2012rahuldayal90No ratings yet
- Searchable SOR E&M 2022-23Document191 pagesSearchable SOR E&M 2022-23Jigar LadhavaNo ratings yet
- Camy Plants: RT Offer LetterDocument1 pageCamy Plants: RT Offer LetterShailesh DeshmukhNo ratings yet