Professional Documents
Culture Documents
Institutionalizing Misinformation - The Dietary Supplement Listing Act of 2022
Uploaded by
Patrick CommettantOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Institutionalizing Misinformation - The Dietary Supplement Listing Act of 2022
Uploaded by
Patrick CommettantCopyright:
Available Formats
PE R S PE C T IV E Physicians Spreading Misinformation on Social Media
ported our right to make judg- “vaccines don’t prevent Covid This article was published on May 18, 2022,
at NEJM.org.
ments about the certification sta- deaths or hospitalizations,” we
tus of physicians to whom we are not dealing with valid profes- 1. Coleman C. License revocation as a re-
grant the credential. sional disagreement; we are deal- sponse to physician misinformation: pro-
ceed with caution. Health Affairs Forefront,
As the ABIM confronts the ing with wrong answers. January 5, 2022 (https://www.healthaffairs
danger of medical misinforma- With nearly 1 million Ameri- .org/do/10.1377/forefront.20211227.966736/).
tion, we recognize that there are cans dead from Covid, and deaths 2. Mello MM. Vaccine misinformation and
the First Amendment — the price of free
many clinical issues on which — some of them clearly prevent- speech. JAMA Health Forum 2022; 3(3):
physicians legitimately hold a able — continuing at a rate of e220732 (https://jamanetwork.com/journals/
spectrum of opinions, all sup- more than 200,000 per year, it jama-health-forum/f ullarticle/2790169).
3. Spreading COVID-19 vaccine misinfor-
ported by evidence; such justifi- has become imperative for our mation may put medical license at risk.
able positions would not make it profession to empower our insti- Washington, DC:Federation of State Medi-
as “distractors” on our exam, nor tutions to signal clearly who is cal Boards, July 29, 2021 (https://www.fsmb
.org/advocacy/news-releases/fsmb-spreading
would they meet our definition — and who is not — providing -covid-19-vaccine-m isinformation-m ay-put
of “false information,” as deter- evidence-based information. We -medical-license-at-risk/).
mined by experts consulting the physicians need to use the insti- 4. Newton W, Baron RJ, Nichols DG. Joint
statement on dissemination of misinforma-
An audio interview literature. A whole tutions we’ve created for profes- tion. Philadelphia:American Board of Inter-
with Dr. Baron is range of statements sional self-regulation to main- nal Medicine, September 9, 2021 (https://
available at NEJM.org with which many — tain public trust by establishing www.abim.org/media-center/press-releases/
joint-statement-on-d issemination-of
or even most — physicians might some recognizable boundaries. -misinformation/).
disagree would therefore not trig- There aren’t always right an- 5. Baron RJ, Emanuel EJ. Politicians should
ger our disciplinary process. On swers, but some answers are not be deciding what constitutes good med-
icine. Stat News, March 7, 2022 (https://
the other hand, when someone clearly wrong. www.statnews.com/2022/03/07/politicians
certified by the ABIM says some- Disclosure forms provided by the authors -should-not-be-deciding-what-constitutes
thing like “the origin of all coro- are available at NEJM.org. -good-medicine/).
nary heart disease is a clearly
From the American Board of Internal Medi- DOI: 10.1056/NEJMp2204813
reversible arterial scurvy” or cine, Philadelphia (R.J.B., Y.D.E.); and Copyright © 2022 Massachusetts Medical Society.
Physicians Spreading Misinformation on Social Media
“children can’t spread Covid” or Brown University, Providence, RI (Y.D.E.).
The Dietary Supplement Listing Act of 2022
Institutionalizing Misinformation — The Dietary Supplement
Listing Act of 2022
Pieter A. Cohen, M.D., Jerry Avorn, M.D., and Aaron S. Kesselheim, M.D., J.D., M.P.H.
B efore the Covid-19 pandemic,
58% of American adults used
vitamins, minerals, botanicals,
sold alongside over-the-counter
medications, but the regulatory
frameworks for these products
more than 2000 hospitalizations
each year are attributed to adverse
effects of dietary supplements.2
live microorganisms, or other differ substantially. Unlike over- Other investigators reported that
dietary supplements to prevent the-counter and prescription the incidence of liver failure re-
or treat various conditions, in- drugs, supplements aren’t vetted lated to dietary supplements in-
cluding viral infections, memory by the Food and Drug Adminis- creased by a factor of 8 between
loss, and heart disease.1 Many tration (FDA) before being intro- the passage of the law currently
people have similarly sought out duced, and their advertising is governing these products, the
supplements to prevent or treat often deceptive. Dietary Supplement Health and
Covid-19, which contributed to The risks associated with sup- Education Act of 1994 (DSHEA),
Americans spending an estimat- plements are real. A Centers for and 2021.3
ed $55 billion on dietary supple- Disease Control and Prevention Little is known about which
ments in 2020. study estimated that about 23,000 of the tens of thousands of sup-
Dietary supplements are often emergency department visits and plement products available for sale
n engl j med 387;1 nejm.org July 7, 2022 3
The New England Journal of Medicine
Downloaded from nejm.org on July 20, 2022. For personal use only. No other uses without permission.
Copyright © 2022 Massachusetts Medical Society. All rights reserved.
PERS PE C T IV E The Dietary Supplement Listing Act of 2022
in the United States pose the information is imported into the products can be described on the
greatest risk to consumers. The new database, even though sup- label as “supporting healthy mem-
FDA is tasked with removing plement labels are known to of- ory” or “supporting healthy brain
dangerous dietary supplements ten be inaccurate. Thousands of function.”
from the market, but it doesn’t products have been found to not The bill wouldn’t stop the
have a systematic approach for contain the advertised ingredients promotion and sale of supple-
monitoring safety, nor can it or to include toxic ingredients not ments that contain dangerous in-
track which supplements are cur- listed on the label. Recent exam- gredients, are poorly manufac-
rently being sold to consumers. ples include vitamin D supple- tured, or are promoted using
Congress is trying to close this ments without the declared quan- prohibited disease claims. The
information gap, but it is off to a tity of vitamin D, potassium FDA would remain unable to
poor start. supplements with more than stop a supplement from being
A bill introduced by Senators twice the listed quantity of po- sold — and listed in the agency’s
Dick Durbin (D-IL) and Mike tassium, berberine supplements database — even if its label lists
Braun (R-IN) in April, the Die- without berberine, and black co- an ingredient banned for use in
tary Supplement Listing Act of hosh supplements that instead supplements, such as the stimu-
2022, would require supplement contained a cheaper plant substi- lant ephedra or the unapproved
manufacturers to provide the FDA tute. In addition to flagging drug phenibut, or if advertising
with a product’s name, ingredi- products with unpredictable vita- for the product includes false
ents, and health claims. For new min doses or undeclared plant and illegal claims, such as that
products, submissions would ingredients, the FDA has identi- it’s a “cure for coronavirus.”
need to be made before the sup- fied more than 1000 supplements The proposed law is unlikely
plement is put on the market. that contained prohibited pre- to improve the quality, effective-
The proposed law would also scription or experimental drugs. ness, or safety of marketed sup-
require the FDA to create and One inevitable outcome of the plements, but it could be a boon
maintain a publicly searchable bill would therefore be to compel for manufacturers. Not only does
database of the estimated 80,000 the FDA to post inaccurate ingre- it decline to place any new re-
supplement products available for dient lists on its website. strictions on the industry, but its
sale in the United States. The bill The legislation also wouldn’t requirements could distract the
is designed, according to Durbin, regulate misleading health claims FDA’s Office of Dietary Supple-
to “protect consumers’ health and made by supplement manufac- ments, which has fewer than
save lives.” The FDA has sought turers. It would continue to allow three dozen employees, from en-
enhanced capabilities for promot- labels to include claims about a forcing the authority it has under
ing supplement safety, suggest- product’s benefits without re- DSHEA to inspect supplement
ing that certain steps, including quiring manufacturers to provide facilities, issue import alerts, and
the establishment of a registry, related evidence to the FDA, so recall hazardous supplements.
could permit the agency to “take long as these claims are phrased From manufacturers’ perspec-
appropriate action to protect con- in terms of effects on the struc- tive, the bill could also help re-
sumers” from dangerous supple- ture or function of the human solve a long-standing public-
ments.4 body, such as “supporting a perception challenge. In the
There is little chance, however, healthy immune system” or “main mainstream media, the supple-
that the proposed law would meet taining normal sugar levels,” and ment industry is often described
these important clinical and pub- don’t mention a specific disease. as operating in the 19th century,
lic health goals; rather, it would Ingredients could also still be free from government oversight.
create the impression of reform marketed as effective after clini- It’s therefore in the industry’s in-
while leaving the current lax reg- cal trials have demonstrated the terest for consumers to believe
ulatory framework largely un- opposite. Ginkgo biloba, for ex- that the FDA is now overseeing
touched. For example, the legis- ample, wasn’t shown to prevent its products. Listing ingredients
lation doesn’t provide the FDA cognitive decline or dementia and specious claims about a
with a mechanism to confirm a when studied in a large, well- product’s benefits on the FDA’s
product’s ingredients before this conducted clinical trial, but these website would implicitly give the
4 n engl j med 387;1 nejm.org July 7, 2022
The New England Journal of Medicine
Downloaded from nejm.org on July 20, 2022. For personal use only. No other uses without permission.
Copyright © 2022 Massachusetts Medical Society. All rights reserved.
PE R S PE C T IV E The Dietary Supplement Listing Act of 2022
agency’s imprimatur to dietary by high-quality clinical trials. all dietary supplements are safe
supplements, even though the These revisions would benefit and accurately promoted, how-
agency would have no new pow- consumers only if members of ever, will require much bolder
ers to ensure their quality, effi- the public could easily confirm legislation that comprehensively
cacy, or safety. that a product was properly reg- reforms DSHEA.
Public health could be better istered with the FDA. The pro- Disclosure forms provided by the authors
served by the creation of a high- posed bill requires that each reg- are available at NEJM.org.
quality registry of all lawfully istered product be assigned a
marketed dietary supplements. unique identifier. If this identifier From Cambridge Health Alliance, Somer-
ville (P.A.C.), and Harvard Medical School
We believe the new bill should be were incorporated into a quick (P.A.C., J.A., A.S.K.) and the Program on
extensively revised to introduce response (QR) code that could be Regulation, Therapeutics, and Law, Divi-
basic safeguards. Congress could scanned before purchase, whether sion of Pharmacoepidemiology and Phar-
macoeconomics, Department of Medicine,
give the FDA the authority to in stores or on the Internet, con- Brigham and Women’s Hospital (J.A.,
read a supplement’s label before sumers could confirm that no A.S.K.), Boston — all in Massachusetts.
registering the product and not prohibited ingredients or disease
register the product if the label claims appeared on the label — This article was published on May 18, 2022,
at NEJM.org.
lists unlawful ingredients or although such registration still
claims. The bill could also be re- wouldn’t provide information 1. Mishra S, Stierman B, Gahche JJ, Po-
vised to permit the FDA to swift- about the product’s efficacy or tischman N. Dietary supplement use among
ly remove products from the reg- safety.5 adults: United States, 2017–2018. NCHS
Data Brief 2021;399:1-8.
istry if the agency determines If advocates of the new legis- 2. Geller AI, Shehab N, Weidle NJ, et al.
that they are hazardous — for lation, including the leading sup- Emergency department visits for adverse
example, if a supplement con- plement trade association, succeed events related to dietary supplements. N Engl
J Med 2015;373:1531-40.
tains toxic levels of selenium or in maneuvering the bill through 3. Ghabril M, Ma J, Patidar KR, et al. Eight-
has been adulterated with a new Congress and into law, it will fold increase in dietary supplement-related
designer drug. The legislation give supplement manufacturers a liver failure leading to transplant waitlisting
over the last quarter century in the United
could require manufacturers to new channel for broadcasting States. Liver Transpl 2022;28:169-79.
submit a certificate of analysis to misinformation: the FDA’s own 4. Department of Health and Human Ser-
ensure that labels are accurate website. By contrast, a thought- vices, Food and Drug Administration. Fiscal
year 2023 justification of estimates for ap-
representations of products’ in- ful revision of the bill could cre- propriations committees. 2022 (https://www
gredients. In addition, to avoid ate a useful resource for regula- .fda.gov/media/157192/download).
misleading consumers, health tors, clinicians, and consumers 5. Kapoor A, Sharfstein JM. Breaking the
gridlock: regulation of dietary supplements
claims submitted to the FDA to confirm that — at a minimum in the United States. Drug Test Anal 2016;8:
shouldn’t be posted publicly on — the ingredients listed on a 424-30.
its website unless the agency supplement’s label are compliant DOI: 10.1056/NEJMp2205675
confirms that they are supported with current law. Ensuring that Copyright © 2022 Massachusetts Medical Society.
The Dietary Supplement Listing Act of 2022
The Portal
The Portal
Suzanne Koven, M.D.
I am a primary care physician
who, for many years, did not
have a primary care physician.
blood pressure and cholesterol, or-
dered mammograms, and placed
a stethoscope over my heart and
Then, when I hit 50, I decided
it was time to get serious about
my own medical care. This is how
Instead, I presented myself annu- lungs, about which I teased him I got serious about my own med-
ally and grudgingly to the Ob/Gyn — though no doubt his skills in ical care: I enlisted a colleague in
who had delivered my babies, auscultation were at least as ad- my internal medicine group to
even long after those babies were vanced as mine in performing serve as my doctor and then ig-
in high school. He checked my pelvic exams. nored most of what she advised
n engl j med 387;1 nejm.org July 7, 2022 5
The New England Journal of Medicine
Downloaded from nejm.org on July 20, 2022. For personal use only. No other uses without permission.
Copyright © 2022 Massachusetts Medical Society. All rights reserved.
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Non Toxic Treatments For CancerDocument46 pagesNon Toxic Treatments For CancerGabriela Vasiliu100% (2)
- What Is Current Good Manufacturing PractDocument4 pagesWhat Is Current Good Manufacturing PractTinsaye HayileNo ratings yet
- Comparing Generic and Innovator Drugs A Review ofDocument16 pagesComparing Generic and Innovator Drugs A Review ofPrachi PandeyNo ratings yet
- Ao 2022 0012Document13 pagesAo 2022 0012Martin Rilloraza Jr.100% (1)
- Cosmetic Product DevelopmentDocument23 pagesCosmetic Product DevelopmentRalp ManglicmotNo ratings yet
- List of AbbreviationsDocument61 pagesList of AbbreviationsrkponrajNo ratings yet
- Regulatory AffairsDocument14 pagesRegulatory AffairsRaul Parga R.No ratings yet
- Case SerenioDocument8 pagesCase SerenioMartin ObiraNo ratings yet
- SarsDocument3 pagesSarsPatrick CommettantNo ratings yet
- Tympanostomy Tubes For Recurrent Otitis Media: Clinical DecisionsDocument3 pagesTympanostomy Tubes For Recurrent Otitis Media: Clinical DecisionsPatrick CommettantNo ratings yet
- ER JusticeDocument3 pagesER JusticePatrick CommettantNo ratings yet
- Omicron VaccinneDocument3 pagesOmicron VaccinnePatrick CommettantNo ratings yet
- Insulin PumpDocument13 pagesInsulin PumpPatrick CommettantNo ratings yet
- Vitamin D SuppDocument3 pagesVitamin D SuppPatrick CommettantNo ratings yet
- Caso Clinico NejDocument9 pagesCaso Clinico NejSilvina MartinezNo ratings yet
- Septic ShockDocument12 pagesSeptic ShockPatrick CommettantNo ratings yet
- Cavitary Lung LesionsDocument9 pagesCavitary Lung LesionsPatrick CommettantNo ratings yet
- SEY-PEN (Package of Essential Non Communicable Diseases)Document85 pagesSEY-PEN (Package of Essential Non Communicable Diseases)Patrick CommettantNo ratings yet
- Question The DiagnosisDocument2 pagesQuestion The DiagnosisPatrick CommettantNo ratings yet
- NEJM Physician ShopDocument2 pagesNEJM Physician ShopZsolt OrbanNo ratings yet
- Perspective: New England Journal MedicineDocument3 pagesPerspective: New England Journal MedicinePatrick CommettantNo ratings yet
- Unexpected Closure: PerspectiveDocument3 pagesUnexpected Closure: PerspectivePatrick CommettantNo ratings yet
- Antiracist ActionDocument3 pagesAntiracist ActionPatrick CommettantNo ratings yet
- Hypertension FinalDocument54 pagesHypertension FinalPatrick CommettantNo ratings yet
- Tympanostomy Tubes For Recurrent Otitis Media: Clinical DecisionsDocument3 pagesTympanostomy Tubes For Recurrent Otitis Media: Clinical DecisionsPatrick CommettantNo ratings yet
- Gout & PseudogoutDocument14 pagesGout & PseudogoutPatrick CommettantNo ratings yet
- The PortalDocument3 pagesThe PortalPatrick CommettantNo ratings yet
- Common Endocrine Conditions: Core Skills and KnowledgeDocument27 pagesCommon Endocrine Conditions: Core Skills and KnowledgePatrick CommettantNo ratings yet
- Omicron Sars-Cov-2 Neutralization From Inactivated and Zf2001 VaccinesDocument4 pagesOmicron Sars-Cov-2 Neutralization From Inactivated and Zf2001 VaccinesPatrick CommettantNo ratings yet
- Diabetic 2 Update Review !!Document82 pagesDiabetic 2 Update Review !!Patrick CommettantNo ratings yet
- Lung and Colorectal Cancer Screening - Ensuring EquityDocument3 pagesLung and Colorectal Cancer Screening - Ensuring EquityPatrick CommettantNo ratings yet
- Diabetes: DR Patrick Commettant Beau Vallon Health CentreDocument26 pagesDiabetes: DR Patrick Commettant Beau Vallon Health CentrePatrick CommettantNo ratings yet
- The Care I NeededDocument3 pagesThe Care I NeededPatrick CommettantNo ratings yet
- Perspective: New England Journal MedicineDocument3 pagesPerspective: New England Journal MedicinePatrick CommettantNo ratings yet
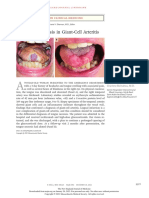
- Tongue Necrosis in Giant Cell ArteritisDocument1 pageTongue Necrosis in Giant Cell ArteritisPatrick CommettantNo ratings yet
- Health EconomicsDocument4 pagesHealth EconomicsPatrick CommettantNo ratings yet
- Artículo HTPDocument16 pagesArtículo HTPSMIBA MedicinaNo ratings yet
- Daftar PustakaDocument7 pagesDaftar PustakaAsih Tri MariniNo ratings yet
- Manual Service Brother 7065Document337 pagesManual Service Brother 7065pheniel1100% (2)
- FDA Warning On DMAADocument2 pagesFDA Warning On DMAARockCenterNBCNo ratings yet
- Related Documents - CREW: Department of State: Regarding International Assistance Offers After Hurricane Katrina: September 2005 DoS CorrespondenceDocument157 pagesRelated Documents - CREW: Department of State: Regarding International Assistance Offers After Hurricane Katrina: September 2005 DoS CorrespondenceCREWNo ratings yet
- 91down JLL Annual Report 2014-15 PDFDocument208 pages91down JLL Annual Report 2014-15 PDFGaurav SiddharthNo ratings yet
- Impurity Profiling of Pharmaceuticals PDFDocument15 pagesImpurity Profiling of Pharmaceuticals PDFvikram kushwahaNo ratings yet
- Notice: Reports and Guidance Documents Availability, Etc.: Expiration Dating of Unit-Dose Repackaged DrugsDocument2 pagesNotice: Reports and Guidance Documents Availability, Etc.: Expiration Dating of Unit-Dose Repackaged DrugsJustia.comNo ratings yet
- Suit Filed Before Judge BlackDocument40 pagesSuit Filed Before Judge BlackCincinnatiEnquirerNo ratings yet
- Creative OutrageDocument416 pagesCreative OutrageKirk TaylorNo ratings yet
- Literature Review On Otc DrugsDocument6 pagesLiterature Review On Otc Drugsafmzsawcpkjfzj100% (1)
- GSK Sotrovimab Fact Sheet For HCP 12222021Document34 pagesGSK Sotrovimab Fact Sheet For HCP 12222021Jillian SmithNo ratings yet
- Bosch XRay WeightMEasurementDocument8 pagesBosch XRay WeightMEasurementsumit_waghmareNo ratings yet
- Plantilla para Plan de Retiro (En Ingles)Document13 pagesPlantilla para Plan de Retiro (En Ingles)Miguel LemusNo ratings yet
- Jubilant Life Sciences Receives ANDA Approval For Rosuvastatin Calcium Tablets (Company Update)Document3 pagesJubilant Life Sciences Receives ANDA Approval For Rosuvastatin Calcium Tablets (Company Update)Shyam SunderNo ratings yet
- Size Shape and Other Physical Attributes of Generic Tablets and CapsulesDocument10 pagesSize Shape and Other Physical Attributes of Generic Tablets and CapsulesrajeebNo ratings yet
- USFDA Guidance For Industry - PSURDocument50 pagesUSFDA Guidance For Industry - PSURErshad Shafi AhmedNo ratings yet
- Emperador 2013Document114 pagesEmperador 2013collieNo ratings yet
- 2-4 What Is A Safety SignalDocument4 pages2-4 What Is A Safety Signalx7w34TNo ratings yet
- Aurobindo Pharma Receives USFDA Approval For Norethindrone Acetate Tablets (Company Update)Document1 pageAurobindo Pharma Receives USFDA Approval For Norethindrone Acetate Tablets (Company Update)Shyam SunderNo ratings yet
- Product Profiling and ManufacturingDocument16 pagesProduct Profiling and ManufacturingPriyanka RawoolNo ratings yet
- Cliantha CorpDocument23 pagesCliantha CorpMaulik PatelNo ratings yet
- Pharmacometric Modeling Article For Drug Development and Marketing Mag 2012Document5 pagesPharmacometric Modeling Article For Drug Development and Marketing Mag 2012Don HeymannNo ratings yet