Professional Documents
Culture Documents
H243-CV16 IgM Insert v6.0
H243-CV16 IgM Insert v6.0
Uploaded by
Neneng Aini KaruniawanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
H243-CV16 IgM Insert v6.0
H243-CV16 IgM Insert v6.0
Uploaded by
Neneng Aini KaruniawanCopyright:
Available Formats
Diagnostic Kit for detection of Positive: Both the quality control area(C) and the test area (T)
uality control area(C) and the test area (T) show red dots.
Anti-Coxsackievirus A16 (CA 16) IgM Antibody Invalid: That a red dot does not present in the quality control area(C) indicates operation faults or
reagents invalidation.
(Colloidal Gold)
INTENDED USE
The test kit is used to detect qualitatively CA 16 IgM antibody in human serum in vitro.
PRINCIPLE
According to the principle of indirect immunoassay, the test utilizes colloidal gold as a mark tracer and
is based on Gold Immunofiltration Assay (GIFA) principle to detect CA 16 IgM in human serum.
MAIN COMPONENTS
Test Device, Colloidal Gold Conjugate, Wash Buffer, Package Insert.
SAMPLE REQUIREMENTS
1.All samples must be centrifuged well immediately prior to using and take the clear serum for ATTENTIONS
detection. 1.The test kit should be transported under temperature of 2 - 8°C.
2.The sample to be detected can be placed for 3 days at 2 - 8°C, or for 3 months at -20°C. 2.This reagent is designed for in vitro diagnosis only, please use within the validity period if opened.
STORAGE AND EXPIRY Different products and different batches of one product can not be cross-used.
1. Store at 2 - 8°C, be valid within 10 months. 3.There must not be time intervals between steps of experiment; The liquid during one step should be
2. Take protective measures in winter and summer to avoid freeze-thawing or high temperature for a added at one time quickly and absorbed completely.
long term. 4. The result observed beyond stated time is invalid.
TEST METHODS 5. The depth of the dot color in quality control area(C) does not indicate the quality of reagents.
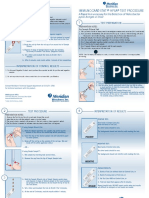
Instruction for Use must be read carefully before taking the test. Allow the required test device to Reagents are valid as long as the dot is clear to identify.
come to room temperature for 30 minutes before use (20 - 30°C). Do not open the inner packaging 6. If the speed of filtration is very slow or even no filtration occurs, please retreat the sample and test
(pouch) until ready. again.
Test Procedure: 7. Serum, including the healthy human serum, is a potential biological hazard substances and the
1. Remove the test device from the pouch and place on a clean and level surface. operator should wear gloves; After testing, items where exposure to serum should be disinfected and
2. Add 2 drops of Wash Buffer into the test window, waiting for the liquid to wet the membrane. then discarded.
3. Add 50 μL of serum into the test window, waiting for the liquid to be absorbed completely. 8. The liquid bottle cover should be tightened immediately after using to avoid contact with air.
4. Add 2 drops of Wash Buffer into the test window, waiting for the liquid to be absorbed completely. 9. Severe chylaemia samples may block the hole of the nitrocellulose membrane, it is recommended
5. Add 3 drops of Colloidal Gold Conjugate into the test window, waiting for the liquid to be absorbed not to be used. Severe chylaemia samples will result in forming red background and affect result
completely. interpretation.
6. Add 3 drops of Wash Buffer into the test window, and interpret the result within 3 minutes MANUFACTURER
immediately after the liquid is absorbed adequately. Qingdao Hightop Biotech Co., Ltd.
LIMITATIONS Address: No.369 Hedong Road, Hi-tech Industrial Development Zone, Qingdao City,Shandong Province,
1. The test kit cannot determine the accurate content of CA 16 IgM antibody. China
2. This assay is a qualitative screening test reagent. The results are only for clinical reference, which Tel: 0086-532-58710705 Web: www.hightopbio.com
are not the only basis for clinical diagnosis and treatment. Fax: 0086-532-58710706 E-mail: sales@hightopbio.com
INTERPRETATION OF RESULTS
Negative: Only the quality control area(C) shows a red dot.
IFU-CA 16 IgM, A/0
You might also like
- HbA1c User Manual-20220413001Document2 pagesHbA1c User Manual-20220413001Pedro RodriguezNo ratings yet
- One Step Anti-HIV (1&2) TestDocument4 pagesOne Step Anti-HIV (1&2) TestGail Ibanez100% (1)
- 13fk10 Hav Igg-Igm (D) Ins (En) CeDocument2 pages13fk10 Hav Igg-Igm (D) Ins (En) CeCrcrjhjh RcrcjhjhNo ratings yet
- SARS-CoV-2 IgM&IgG Rapid Test PDFDocument2 pagesSARS-CoV-2 IgM&IgG Rapid Test PDFfatmaNo ratings yet
- v1 Panbio COVID 19 Ag Nasal AsymptomaticSeDocument27 pagesv1 Panbio COVID 19 Ag Nasal AsymptomaticSeIvanNo ratings yet
- v2 Panbio COVID-19 Ag Nasal AsymptomaticSeDocument158 pagesv2 Panbio COVID-19 Ag Nasal AsymptomaticSesilvia micaela terrazas silesNo ratings yet
- v2 Panbio COVID-19 Ag Nasal IFU 41FK11 MultDocument132 pagesv2 Panbio COVID-19 Ag Nasal IFU 41FK11 MultAndres Mendoza AngelesNo ratings yet
- HCV Package InsertDocument1 pageHCV Package Insertgitadevistha23No ratings yet
- Manual AdLeptospiraIgMIgGCardDocument2 pagesManual AdLeptospiraIgMIgGCardMatibar RahmanNo ratings yet
- Mybiosource: Covid-19 Igg/Igm Antibody Assay KitDocument3 pagesMybiosource: Covid-19 Igg/Igm Antibody Assay KitMatibar RahmanNo ratings yet
- IFU For SARS-CoV-2 Ag Diagnostic KitDocument3 pagesIFU For SARS-CoV-2 Ag Diagnostic KitLeonel OjedaNo ratings yet
- v3 Panbio COVID-19 Ag Nasopharyngeal IFU BoDocument19 pagesv3 Panbio COVID-19 Ag Nasopharyngeal IFU BoAinul KhakimNo ratings yet
- (A Rapid Test For Detection of Typhoid Fever) : Typhidot Rapid Igg/Igm (Combo)Document2 pages(A Rapid Test For Detection of Typhoid Fever) : Typhidot Rapid Igg/Igm (Combo)Anonymous yfIFkVUANo ratings yet
- Binax Product InsertDocument96 pagesBinax Product Insertuber6791No ratings yet
- HBSAG Rapid Test 2Document6 pagesHBSAG Rapid Test 2Charlotte OhNo ratings yet
- RAT Investigatory ProjectDocument11 pagesRAT Investigatory ProjectanoopstudieNo ratings yet
- Vista Hepatitis B Surface AntigenDocument3 pagesVista Hepatitis B Surface Antigenadam parthen kaneNo ratings yet
- Male Fertility Sperm Test For Home UseDocument2 pagesMale Fertility Sperm Test For Home UseClaudia PaihoNo ratings yet
- Ctni Rapid Quantitative Test: Limitations of ProcedureDocument2 pagesCtni Rapid Quantitative Test: Limitations of ProcedureTrần Thanh Viện100% (1)
- IFU For SARS-CoV-2 Ab Diagnostic KitDocument3 pagesIFU For SARS-CoV-2 Ab Diagnostic KitLeonel OjedaNo ratings yet
- Typhoid IgG-IgjjDocument2 pagesTyphoid IgG-IgjjChaudhary HarshNo ratings yet
- 2-Ifu-Test Kit PDFDocument3 pages2-Ifu-Test Kit PDFAlma AparicioNo ratings yet
- Avitex CRP CE Globe A5 V4Document2 pagesAvitex CRP CE Globe A5 V4MuhammadSahidNo ratings yet
- Widal 8x5mlDocument2 pagesWidal 8x5mlLễ Phan ThịNo ratings yet
- SARS-CoV2 (COVID-19) IgG IgM C20200203Document2 pagesSARS-CoV2 (COVID-19) IgG IgM C20200203Mönica YauriNo ratings yet
- 7 IFU-EV71CA16 IgM antibody combo test cassette - Immunochromatography - 22.6.9 - 1 - -修订已发研发Document2 pages7 IFU-EV71CA16 IgM antibody combo test cassette - Immunochromatography - 22.6.9 - 1 - -修订已发研发Neneng Aini KaruniawanNo ratings yet
- HBsAg 40 Tests Ing Rev. 07Document2 pagesHBsAg 40 Tests Ing Rev. 07Mayra VallesNo ratings yet
- SARS-CoV-2 Antigen Instruction-CE (Only Nasal Swab, Professional Use)Document2 pagesSARS-CoV-2 Antigen Instruction-CE (Only Nasal Swab, Professional Use)camiloviviNo ratings yet
- IFU For SARS-CoV-2 IgM Diagnostic KitDocument3 pagesIFU For SARS-CoV-2 IgM Diagnostic KitLeonel OjedaNo ratings yet
- EUA Autobio Rapid Ifu3Document13 pagesEUA Autobio Rapid Ifu3Elaila ArcaNo ratings yet
- Dengue Igm Elisa: For The Quantitative Determination of Igm-Class Antibodies To Dengue Virus in SerumDocument9 pagesDengue Igm Elisa: For The Quantitative Determination of Igm-Class Antibodies To Dengue Virus in SerumTanveerNo ratings yet
- Fob Cassette PDFDocument2 pagesFob Cassette PDFHeang ChanryNo ratings yet
- Informatii Despre Test enDocument14 pagesInformatii Despre Test enCorina1997gmail.com Cazarinov CorinaNo ratings yet
- Procedure Card ImmunoCard Stat HpSADocument2 pagesProcedure Card ImmunoCard Stat HpSAvijayaNo ratings yet
- Rat HbA1c Assay v4Document6 pagesRat HbA1c Assay v4Amos Babu JettiNo ratings yet
- Rapid Anti-HIV (1&2) Test: Reagents and Materials SuppliedDocument4 pagesRapid Anti-HIV (1&2) Test: Reagents and Materials Suppliedweli81_131308225No ratings yet
- Sars-Cov-2 Igg Elisa Kit (DEIASL019) : Product InformationDocument6 pagesSars-Cov-2 Igg Elisa Kit (DEIASL019) : Product InformationТатьяна ИсаеваNo ratings yet
- COVID-19 ANTIGEN SKYTEST BrochureDocument1 pageCOVID-19 ANTIGEN SKYTEST BrochurerianNo ratings yet
- Chikungunya IgM Combo Rev DDocument2 pagesChikungunya IgM Combo Rev DDaniel LaraNo ratings yet
- Hav Igg Igm S P Mym Ji 076 077Document2 pagesHav Igg Igm S P Mym Ji 076 077Ruben DuranNo ratings yet
- Id Now Covid-19: For Use Under An Emergency Use Authorization (EUA) OnlyDocument21 pagesId Now Covid-19: For Use Under An Emergency Use Authorization (EUA) OnlyMichael WoodsNo ratings yet
- TMCAC1J9 Tumor Marker Control Insert Rev0Document1 pageTMCAC1J9 Tumor Marker Control Insert Rev0Edgar GalvánNo ratings yet
- COVID-19 Antigen Test Kit-Surge MedcialDocument16 pagesCOVID-19 Antigen Test Kit-Surge Medcialwalter sandoNo ratings yet
- Uni-Gold HIV Rapid TestDocument1 pageUni-Gold HIV Rapid TestEmmanuel SamuelNo ratings yet
- AVOX July2011Document6 pagesAVOX July2011Kriss JonesNo ratings yet
- 2-4.IFU-SARS-CoV-2 Saliva Antigen Rapid Test (Colloidal Gold Method)Document2 pages2-4.IFU-SARS-CoV-2 Saliva Antigen Rapid Test (Colloidal Gold Method)sunny niNo ratings yet
- IFU Wondfo SARS CoV 2 Antibody Test (Lateral Flow Method) PDFDocument2 pagesIFU Wondfo SARS CoV 2 Antibody Test (Lateral Flow Method) PDFEndrio R HartonoNo ratings yet
- CareStart Training Slides 002 719655 7Document18 pagesCareStart Training Slides 002 719655 7Luis SanchezNo ratings yet
- Instant-View: H. Pylori Serum Cassette TestDocument2 pagesInstant-View: H. Pylori Serum Cassette TestGrace ValenciaNo ratings yet
- Immulite 2000 Top: 1. Principle: The IMMULITE 2000 Automated Immunoassay Analyzer IsDocument3 pagesImmulite 2000 Top: 1. Principle: The IMMULITE 2000 Automated Immunoassay Analyzer IsFaryalBalochNo ratings yet
- Feline Calicivirus Ag Test: Cat No.: W81201Document1 pageFeline Calicivirus Ag Test: Cat No.: W81201Andri saikNo ratings yet
- GEM OPL Training SFHN VersionDocument39 pagesGEM OPL Training SFHN Versiongoldenpower engNo ratings yet
- A Rapid Test For Detection of Dengue FeverDocument2 pagesA Rapid Test For Detection of Dengue FeverYvette TiongsonNo ratings yet
- UIBC Control: Package Size Intended UseDocument19 pagesUIBC Control: Package Size Intended Usetech yuva cscNo ratings yet
- Widal TestDocument11 pagesWidal TestlialestariNo ratings yet
- H Pilory HecesDocument2 pagesH Pilory HecesKarla SotoNo ratings yet
- Analizador GP Getein 1100 ManualDocument2 pagesAnalizador GP Getein 1100 Manualbiomedico international0% (1)
- Quality Control in Microbiology and SerologyDocument10 pagesQuality Control in Microbiology and SerologyW.F KareemNo ratings yet
- Ins0079 - Snapshot Hy Lite Rev CDocument1 pageIns0079 - Snapshot Hy Lite Rev CRustam AlyaskarovNo ratings yet
- Guidelines For Applying For Ethical Clearance of Research Projects From NBCDocument2 pagesGuidelines For Applying For Ethical Clearance of Research Projects From NBCNeneng Aini KaruniawanNo ratings yet
- Reg PER1Document2 pagesReg PER1Neneng Aini KaruniawanNo ratings yet
- Guidelines On Clinical Trial Applications For ApplicantsDocument63 pagesGuidelines On Clinical Trial Applications For ApplicantsNeneng Aini KaruniawanNo ratings yet
- Sa GCP 2020 FinalDocument71 pagesSa GCP 2020 FinalNeneng Aini KaruniawanNo ratings yet
- Performance Evaluation of Registry IRIS and CCADocument17 pagesPerformance Evaluation of Registry IRIS and CCANeneng Aini KaruniawanNo ratings yet
- Requirements For CRO ApprovalDocument3 pagesRequirements For CRO ApprovalNeneng Aini KaruniawanNo ratings yet
- Open Label Extension Study Guidlines 10jul2017Document1 pageOpen Label Extension Study Guidlines 10jul2017Neneng Aini KaruniawanNo ratings yet
- Informed Consent Document Adolescents Timothy Holman, MADocument7 pagesInformed Consent Document Adolescents Timothy Holman, MANeneng Aini KaruniawanNo ratings yet
- Seroepidemiology of Human Enterovirus 71, Singapore: The StudyDocument3 pagesSeroepidemiology of Human Enterovirus 71, Singapore: The StudyNeneng Aini KaruniawanNo ratings yet
- The Effect of The E-Patuh Application On HIV/Aids Patients' Adherence in Consuming AntiretroviralDocument9 pagesThe Effect of The E-Patuh Application On HIV/Aids Patients' Adherence in Consuming AntiretroviralNeneng Aini KaruniawanNo ratings yet
- 7 IFU-EV71CA16 IgM antibody combo test cassette - Immunochromatography - 22.6.9 - 1 - -修订已发研发Document2 pages7 IFU-EV71CA16 IgM antibody combo test cassette - Immunochromatography - 22.6.9 - 1 - -修订已发研发Neneng Aini KaruniawanNo ratings yet
- Semi-Final Examination in Technology and Livelihood Education (FOOD PROCESSING) For Grade 8Document4 pagesSemi-Final Examination in Technology and Livelihood Education (FOOD PROCESSING) For Grade 8CYNTHIA GALEONNo ratings yet
- $X, XXX $X, XXX: Product Name Product NameDocument1 page$X, XXX $X, XXX: Product Name Product NameRidwan AgustiantoNo ratings yet
- All About The T-CON Board: LED/LCD TV T-CON & Screen Panel Repair GuideDocument21 pagesAll About The T-CON Board: LED/LCD TV T-CON & Screen Panel Repair GuideRafael Fernández100% (8)
- Radio Detection and Ranging (RADAR)Document30 pagesRadio Detection and Ranging (RADAR)Tulshinarayan GiriNo ratings yet
- AdrenaLinn III Users Manual v302 5-14-08Document88 pagesAdrenaLinn III Users Manual v302 5-14-08totulsaunimic100% (1)
- Samsung Horticulture Technical Brochure 190627Document16 pagesSamsung Horticulture Technical Brochure 190627Stiven AndrewNo ratings yet
- MAIN Electrical Parts List: Design LOC Sec-Code DescriptionDocument12 pagesMAIN Electrical Parts List: Design LOC Sec-Code DescriptionAndroid Schematics and CircuitsNo ratings yet
- Ielts Speaking 200 Sample Answers Ielts 2Document41 pagesIelts Speaking 200 Sample Answers Ielts 2teste858585No ratings yet
- Agua Vaporizador WB-4500Document3 pagesAgua Vaporizador WB-4500Caterine Jara CeaNo ratings yet
- Lec 23-24 Euler MethodDocument36 pagesLec 23-24 Euler MethodEngineer Inside33% (3)
- Method Statement - Handover NotesDocument5 pagesMethod Statement - Handover NotesHamza NadeemNo ratings yet
- Hugo Riemann - Harmony SimplifiedDocument94 pagesHugo Riemann - Harmony SimplifiedDewdman42100% (1)
- Advanced Engineering MathematicsDocument21 pagesAdvanced Engineering MathematicsSilambarasan VeluchamyNo ratings yet
- Chapter 2 Molecules of LifeDocument67 pagesChapter 2 Molecules of LifeNatalie GraceNo ratings yet
- Hagerman - Training The Energy SystemsDocument8 pagesHagerman - Training The Energy Systemsmkn1214100% (1)
- Trimble Zeiss 330 X EnglDocument256 pagesTrimble Zeiss 330 X Englvlad_2121100% (1)
- Guidelines SOP For Monitoring and Compliance of All Safety Norms On The HighwaysDocument15 pagesGuidelines SOP For Monitoring and Compliance of All Safety Norms On The HighwaysBilal A BarbhuiyaNo ratings yet
- Air Muscle PresentationDocument23 pagesAir Muscle PresentationNm5793No ratings yet
- Improvement of Voltage Profile Through The Optimal Placement of FACTS Using L-Index MethodDocument5 pagesImprovement of Voltage Profile Through The Optimal Placement of FACTS Using L-Index MethodRavishankar KankaleNo ratings yet
- Pooping and Eating PDFDocument2 pagesPooping and Eating PDFIsabel Christina Gonzalez MoralesNo ratings yet
- Technical ProposalDocument4 pagesTechnical ProposalolescootNo ratings yet
- PBXs C4 Sem10Document17 pagesPBXs C4 Sem10justinshine11No ratings yet
- Lenses: Lesson 5 (3 Hours)Document19 pagesLenses: Lesson 5 (3 Hours)DonnaNo ratings yet
- SMPS TopologyDocument4 pagesSMPS TopologyGrigore ManNo ratings yet
- FORScan Programming Modules Acronyms-AbbreviationsDocument3 pagesFORScan Programming Modules Acronyms-AbbreviationsHajirashid26100% (1)
- Concept of Tensors Deformation of A Body Stress Equilibrium Equations Constitutive Equations Principles From Work and Energy Homework ReferencesDocument19 pagesConcept of Tensors Deformation of A Body Stress Equilibrium Equations Constitutive Equations Principles From Work and Energy Homework Referencess2rajiNo ratings yet
- Food Tests 1. Carbohydrates (Sugars and Starches)Document3 pagesFood Tests 1. Carbohydrates (Sugars and Starches)nmrasaNo ratings yet
- Chemical Constituents and Biological Activities of Cichorium Intybus LDocument6 pagesChemical Constituents and Biological Activities of Cichorium Intybus Lreza rezaieNo ratings yet
- Projects Based On Audino DC Motor ControlDocument15 pagesProjects Based On Audino DC Motor Controlnagasaikiran ponnapalli0% (1)
- Mathematical Methods MT2017: Problems 2: John - Magorrian@physics - Ox.ac - UkDocument2 pagesMathematical Methods MT2017: Problems 2: John - Magorrian@physics - Ox.ac - UkRoy VeseyNo ratings yet