0% found this document useful (0 votes)
636 views36 pagesChapter 2 Material Balance Fazliedited
Here are the steps to solve this problem:
1. Draw a flow diagram showing the inputs and outputs of the mixing tank
2. Select units (kg and % w/w assumed)
3. Write the general material balance equations for invert sugars, water and solids
4. Calculate the amount of water needed based on the given invert sugar concentration in the output
5. Check material balances for all components
Let me know if you need help with any of the calculations.
Uploaded by
Thivya KarthigayanCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
636 views36 pagesChapter 2 Material Balance Fazliedited
Here are the steps to solve this problem:
1. Draw a flow diagram showing the inputs and outputs of the mixing tank
2. Select units (kg and % w/w assumed)
3. Write the general material balance equations for invert sugars, water and solids
4. Calculate the amount of water needed based on the given invert sugar concentration in the output
5. Check material balances for all components
Let me know if you need help with any of the calculations.
Uploaded by
Thivya KarthigayanCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Introduction to Material Balance: Introduces the basic principles of material balance, establishing the foundational idea that mass in biological systems is conserved.
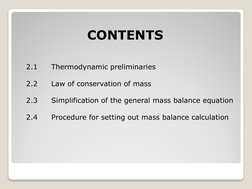
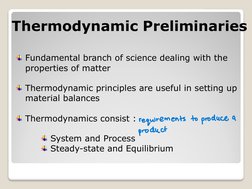
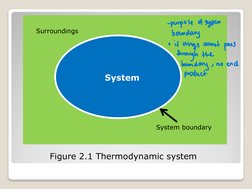
- Thermodynamic Preliminaries: Discusses the role of thermodynamics in material balances, including fundamental principles necessary for understanding system and process behavior.
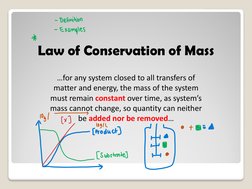
- Law of Conservation of Mass: Explores the concept that mass is conserved in closed systems and the implications of this principle on mass balance calculations.
- General Mass Balance Equation: Details the general mass balance equation, providing examples to illustrate its application in systems analysis.
- Simplification of the General Mass Balance Equation: Describes how to simplify the mass balance equation for steady state processes, focusing on eliminating the accumulation term.
- Procedure for Setting Out Mass Balance Calculation: Outlines a step-by-step procedure for performing material balance calculations, including drawing flow diagrams and making assumptions.
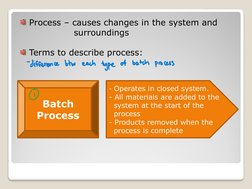
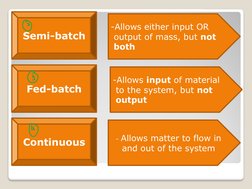
- Example Applications in Mass Balance Calculations: Provides practical examples of mass balance calculations, including continuous filtration and batch mixing scenarios with detailed solutions.