Professional Documents
Culture Documents
CM 91 469
Uploaded by
Houda El MoufidOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CM 91 469
Uploaded by
Houda El MoufidCopyright:
Available Formats
DOI: 10.
15386/cjmed-951 Case Report
CLINICAL AND ENDOSCOPIC MANIFESTATIONS OF
GASTROINTESTINAL AMYLOIDOSIS: A CASE SERIES
ANDREE HERMES KOOP, OMAR Y MOUSA, MING-HSI WANG
Gastroenterology and Hepatology Department, Mayo Clinic Florida, United States
Abstract
Gastrointestinal (GI) amyloidosis is rare and has varying clinical and endoscopic
presentations. In this case series, we present three patients with primary systemic
amyloid-light chain (AL) amyloidosis with GI involvement and complications of GI
bleeding. We also provide a brief review of the literature, including clinical presentation,
endoscopic findings, pathology, and management of GI amyloidosis. The endoscopic
findings of GI amyloidosis can vary, including friable mucosa with erosions, ulcers, and
submucosal hematomas or mucosal thickening with polypoid protrusions. The endoscopic
findings may correlate with the pathologic deposition of amyloid fibrils. Treatment of
GI amyloidosis is generally focused on management of the underlying condition and
supportive care. Gastroenterologists should be familiar with the endoscopic findings as
they may be the first suggestion of disease and allow for definitive diagnosis.
Keywords: amyloid, amyloidosis, gastrointestinal hemorrhage
Introduction bone marrow. He underwent a second autologous stem cell
Gastrointestinal (GI) amyloidosis with both transplant with melphalan therapy.
symptoms and biopsy-proven involvement of the intestinal Two weeks after repeat transplantation, the patient
tissue is uncommon, although GI-related symptoms are developed acute abdominal pain and diarrhea. White blood
reported variably in primary (AL) amyloidosis (8%-60%) cell count was 19,000 cells/L. Computed tomography
and secondary amyloid A (AA) amyloidosis (10%-70%) imaging of the abdomen revealed pancolitis; testing for
[1,2]. We present a case series of 3 patients with primary Clostridium dificile was negative. Flexible sigmoidoscopy
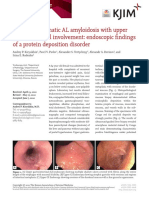
systemic AL amyloidosis with GI involvement and showed severe colitis with diffuse mucosal ulceration (Figure
bleeding. Following the case presentation, we provide a 1). Immunostaining was negative for cytomegalovirus, and
review of GI amyloidosis, including clinical presentation, pathology was positive for amyloid deposition. He was
endoscopic findings, pathology, and treatment. treated with a 7-day course of oral budesonide and required
Case Series total parenteral nutrition for one week, after which, his
Patient characteristics for cases 1, 2, and 3 are listed symptoms improved and his diet was advanced. He had
in Table 1. clinical remission of amyloidosis on bone marrow biopsy 3
Case 1 months following autologous stem cell transplantation, with
A 53-year-old man was evaluated for exertional resolution of abdominal pain and diarrhea.
shortness of breath, and cardiac magnetic resonance imaging
was suspicious for cardiac amyloidosis. A fat aspirate
confirmed AL λ amyloidosis, and a bone marrow biopsy
showed 7% atypical plasma cells. The patient underwent
autologous stem cell transplantation for AL amyloidosis with
complete remission. Six years later, he developed symptoms of
constipation and neuropathy. Esophagogastroduodenoscopy
(EGD) revealed erythematous mucosa in the gastric antrum,
with amyloid deposition on pathology. Fat aspirate and
bone marrow biopsy also revealed amyloid involvement
concerning for disease relapse, with 5% plasma cells in the
Manuscript received: 20.12.2017
Received in revised form: 06.06.2018 Figure 1. Sigmoidoscopy showing severe colitis with
Accepted: 20.06.2018
Address for correspondence: koop.andree@mayo.edu diffuse mucosal ulcerations in the whole examined colon.
Clujul Medical Vol. 91, No. 4, 2018: 469-473 469
Case Report
Table 1.
Case #, Age Time to detection of Gastrointestinal Endoscopic findings Pathology from Management of GI
and Gender GI Amyloidosis symptoms endoscopic biopsy manifestations
#1 6 years after Acute abdominal Sigmoidoscopy; severe Severe active colitis Symptoms subsided after
53 M diagnosis of systemic pain and diarrhea colitis with diffuse mucosal with focal vascular second ASCT
amyloidosis ulceration amyloidosis,
Negative for CMV
EGD; antral erythema
Reactive and erosive
gastropathy
#2 Time of diagnosis of Nausea and Colonoscopy; large polyp in Tubulovillus Anti-nausea medications,
74 F systemic amyloidosis vomiting, upper the sigmoid colon adenoma and colonic hospice care
GI bleeding amyloidosis
EGD; granular gastric
mucosa, erythema and
edema of the gastric antrum, Chronic active
pylorus, and duodenal gastritis, gastric
bulb with gastric outlet and duodenal
obstruction amyloidosis
#3 9 years after Recurrent GI EGD; severe erythematous, Chronic Red blood cell transfusion,
72 M diagnosis of systemic bleeding with friable gastric and duodenal inflammation with proton pump inhibitor,
amyloidosis melena mucosa amyloidosis aminocaproic acid
Case 2 patient was started on treatment with ibrutinib; however,
A 74-year-old woman presented to her primary care she developed early satiety, nausea and coffee grounds
physician with fatigue, 20-pound weight loss, and iron- emesis two weeks later. EGD revealed diffuse, friable,
deficiency anemia. A colonoscopy revealed a large sigmoid edematous, polypoid protrusion of the duodenal mucosa
mass, and pathology demonstrated a tubulovillous adenoma with gastric outlet obstruction (Figure 2); biopsy confirmed
with amyloid deposition. Bone marrow biopsy confirmed AL amyloidosis (Figure 3a and 3b). She was initially
AL κ amyloidosis with Waldenström (No ‘s per Dorland’s managed with a nasogastric tube and anti-emetics. Given
and AMA.) macroglobulinemia. An echocardiogram was her poor clinical condition and prognosis she was referred
suspicious for cardiac involvement of amyloidosis. The to hospice care and expired 5 months later.
Figure 2. EGD showing friable, polypoid protrusion
of the duodenal mucosa.
a b
Figure 3. a and b, histopathology showing duodenal amyloidosis. Figure 3a illustrates amyloid deposition under Congo
red staining, and Figure 3b shows apple-green birefringence under polarized light microscopy.
470 Clujul Medical Vol. 91, No. 4, 2018: 469-473
Case Report
Case 3 in tissue as rigid, nonbranching fibrils approximately 10
A 72-year-old man presented with edema, and nm in diameter [5]. The distinctive appearance of amyloid
was diagnosed with nephrotic syndrome. A renal biopsy on polarized light microscopy, and the gold standard for
confirmed AL λ amyloidosis, and evaluation showed no diagnosis, is apple-green birefringence under Congo red
evidence of other organ involvement. The patient was treated staining. Tissue deposition of these proteins causes direct
with melphalan and autologous stem cell transplantation. tissue damage and disruption of tissue architecture, leading
Seven years later, he had worsening of his kidney function, to organ dysfunction [3,6].
and repeat kidney biopsy revealed relapsed amyloidosis. Organs are variably involved depending on the
He was treated with bortezomib, without improvement in type, with the heart and kidneys most commonly involved
renal function, and eventually required hemodialysis. in AL amyloidosis, and the heart and nervous system most
Two years after relapse of amyloidosis, the patient commonly involved in ATTR [7]. The most common site of
developed recurrent episodes of melena requiring frequent GI mucosal infiltration is the second part of the duodenum,
blood transfusions. EGD showed multiple friable and followed by the stomach, colorectum, and esophagus [8].
erythematous polypoid protrusions of the gastric and In a single-center retrospective study of 2,334 patients
duodenal mucosa with confirmation of amyloid deposition with amyloidosis, 3% had biopsy-proven GI tract amyloid
on pathology (Figure 4). Colonoscopy revealed several involvement, of which, 80% had systemic amyloidosis and
small tubular adenomas, but otherwise, no obvious source 20% had no other organ involvement.(9) GI symptoms
of bleeding. Deep bowel enteroscopy did not reveal are estimated to occur in up to 60% of patients with AL
an alternative etiology for bleeding. His bleeding was amyloidosis and 70% with AA amyloidosis [1,2]. Despite
determined to be from diffusely erythematous gastric and the high prevalence of GI symptoms, biopsy-proven GI
duodenal mucosa in the setting of chronic renal failure involvement is less common (3%-7%) [6,9]. Involvement
and thrombocytopenia. Due to frequent melena, he was of the GI tract in AL amyloidosis is defined by the presence
dependent on blood transfusions several times per week to of GI symptoms and direct biopsy verification [10]. There
maintain a hemoglobin level higher than 7 g/dL. He was are no standardized guidelines for diagnosis and staging for
treated with aminocaproic acid with mild improvement in the other systemic and localized amyloidoses, and clinicians
bleeding; however ultimately, hospice care was pursued. often define organ involvement per the guidelines for AL
amyloidosis.
The presenting symptoms of GI amyloidosis are
variable and nonspecific, including weight loss, early
satiety, GI bleeding, and abdominal pain. Patients may
have malabsorption characterized by diarrhea, steatorrhea,
hypoalbuminemia, or other dysmotility-type symptoms,
including nausea and heartburn [9,11,12]. The clinical signs
and symptoms of hepatic amyloidosis are broad, ranging
from findings of hepatomegaly and elevated liver function
tests to stigmata of portal hypertension and hepatic failure
[13]. Within the GI tract, amyloid deposition occurs in the
muscularis mucosa close to the nerves and vasculature,
leading to increased vessel fragility, decreased peristalsis,
decreased compliance of the gut wall, and associated
symptoms [14-16].
The endoscopic findings of amyloidosis are
Figure 4. Severe erythematous duodenopathy. variable and may correlate with the clinical symptoms
and findings on histopathology. Characteristic endoscopic
findings in AL amyloidosis include multiple polypoid
Discussion protrusions and thickening of the valvulae conniventes.
Amyloidosis is characterized by the misfolding of Accordingly, presenting symptoms are constipation or
extracellular proteins, called amyloid fibrils, with tissue signs of mechanical obstruction or pseudo-obstruction.
deposition of insoluble, toxic protein aggregates in the Additional findings are mucosal erosions, ulcerations, and
extracellular spaces of organs and tissues [3,4]. The four main submucosal hematomas, which may be a distinctive finding
systemic types include AL amyloidosis; AA, or secondary for AL amyloid involvement of the GI tract with bleeding
amyloidosis; familial amyloidosis (ATTR), characterized [1]. In our case series, the first patient demonstrated colitis
by mutations in the precursor protein transthyretin; and with diffuse mucosal ulceration, which improved following
β2-microglobulin amyloidosis associated with end-stage repeat autologous stem cell transplantation. The other two
renal disease and dialysis [4]. The amyloid fibrils deposit patients had polypoid protrusions with friable mucosa.
Clujul Medical Vol. 91, No. 4, 2018: 469-473 471
Case Report
Endoscopically, AA amyloidosis presents as a involvement [8]. Capsule endoscopy can be considered
fine granular appearance of the mucosa, with associated for evaluation of patients with a suspected small bowel
symptoms of diarrhea, malabsorption, and GI bleeding [17]. source of bleeding from amyloid, and negative EGD and
Amyloid deposition is predominantly in the lamina propria colonoscopy [22].
[17]. In AL amyloidosis, amyloid deposition is generally Treatment of amyloidosis is aimed at reducing the
found in the outer coat of small- and medium-sized blood production of amyloidogenic protein to prevent further
vessels, with parenchymal deposition in the muscle layers. growth of amyloid deposits and organ dysfunction. Early
In ATTR and AA amyloidosis, amyloid deposition occurs diagnosis is important to start treatment and prevent
in the inner coat of small blood vessels, and parenchymal progression [4]. Treatment of AL amyloidosis is directed
deposition occurs primarily in the mucosa [18]. at eradicating the underlying plasma cell dyscrasia with
In a retrospective study of 20 patients with chemotherapy. High-dose melphalan and autologous stem
symptomatic GI amyloidosis, submucosal infiltration cell transplantation has shown survival benefit, despite a
correlated with a granular or thickened mucosa on high treatment-related mortality of 15% [25]. Several novel
endoscopy. In patients with infiltration of the lamina agents, including the proteasome inhibitor, bortezomib,
propria (mucosa), superficial lesions predominated, are under evaluation in combination with dexamethasone
including ulcers, erosions, friability, and edema. Although [26]. Similarly in AA amyloidosis, treatment is aimed at
the location of amyloid deposition on pathology correlated decreasing AA serum levels by suppression or eradication of
with the endoscopic findings, there was no correlation with the underlying inflammatory disease [4]. Currently, the only
the specific type of amyloid [19]. established treatment of patients with ATTR amyloidosis is
The location of involvement along the GI tract and liver transplantation, which removes the source of mutated
within the gut wall varies among the types of amyloidosis. transthyretin from the circulation [27]. Treatment for β2-
Overall, AL amyloidosis is the most common form within microglobulin amyloidosis is kidney transplantation [4].
the GI tract. In a retrospective study of 663 biopsies Systemic amyloidosis generally has a grave prognosis as
of the GI tract demonstrating amyloid deposition, most cases are diagnosed late into the disease course with
mucosal involvement, as compared to submucosal multi-organ dysfunction, limiting therapeutic interventions.
involvement, was more common in AA and AL κ Diagnosis is often delayed due to the nonspecific presenting
amyloidosis, less common in AL λ amyloidosis, and rare signs and symptoms of this disease, and its insidious
in ATTR amyloidosis. AL κ and ATTR amyloidosis were progression [1]. Two studies have found the median delay
distributed more commonly in the upper GI tract, and AL from onset of symptoms to diagnosis was 7 months [11,12].
λ and ATTR amyloidosis in the large intestine. Based on The prognosis of AL amyloidosis is poor, with a reported
these findings, the authors proposed that endoscopists use median survival between 12 and 18 months, depending on
this information to guide selection of the biopsy site when severity and number of organs involved [28,29].
amyloidosis is suspected [20]. Treatment of GI involvement of amyloidosis is
Any amyloid-containing biopsy of the GI tract is directed at management and amelioration of symptoms [2].
suitable for diagnosis of amyloidosis. As the symptoms It is important to address adequate hydration and nutritional
of amyloidosis are often nonspecific, endoscopists should intake, particularly in patients with malabsorption. For
have a high clinical suspicion when performing endoscopy disabling dysmotility-related symptoms, total parenteral
as diagnosis can be confirmed with pathology. Diagnosis nutrition can be considered [2]. Treatment with lomotil or
of amyloidosis was confirmed via colonoscopy in Case loperamide may be helpful in patients with diarrhea, and
2. Biopsy from the GI tract is accessible, and may negate metoclopramide in patients with early satiety. Antiemetics,
further need for invasive testing, including cardiac or renal such as prochlorperazine, promethazine, and ondansetron
biopsy. Pathologic evaluation for amyloidosis with Congo hydrochloride, may be helpful in patients with nausea and
red staining may not be routine practice at all medical vomiting [9].
centers, which again requires high clinical suspicion. In summary, symptomatic and biopsy-proven GI
GI bleeding is rare as the initial presentation or sole involvement of amyloidosis is not commonly reported,
manifestation of amyloidosis, and may be obscure, overt, and has varying clinical and endoscopic presentations. We
and even life-threatening. Deposition of amyloid causes present a case series of three patients with GI amyloidosis
vascular fragility, which may produce submucosal bleeding and associated GI bleeding. Common endoscopic findings
and hematoma formation. Endoscopic and surgical in GI amyloidosis with bleeding include friable mucosa
treatment of bleeding from GI amyloidosis is difficult with ulcerations and submucosal hematomas. Treatment
given the widespread nature of the disease [23]. Surgical of GI bleeding from amyloidosis is difficult, given the
resection of an expanding gastric submucosal hematoma widespread nature of the disease and bleeding. The
has been reported in the literature [24]. Generally, treatment prognosis of GI amyloidosis is often poor and related to the
is directed at the underlying condition. The second part severity of the underlying illness.
of the duodenum is the most common site of GI amyloid
472 Clujul Medical Vol. 91, No. 4, 2018: 469-473
Case Report
References 16. Hirschfield GM. Amyloidosis: a clinico-pathophysiological
1. James DG, Zuckerman GR, Sayuk GS, Wang HL, Prakash synopsis. Semin Cell Dev Biol. 2004;15(1):39-44.
C. Clinical recognition of Al type amyloidosis of the luminal 17. Tada S, Iida M, Yao T, Kawakubo K, Yao T, Okada M, et al.
gastrointestinal tract. Clin Gastroenterol Hepatol. 2007;5(5):582- Endoscopic features in amyloidosis of the small intestine: clinical
588. and morphologic differences between chemical types of amyloid
2. Petre S, Shah IA, Gilani N. Review article: gastrointestinal protein. Gastrointest Endosc. 1994;40(1):45-50.
amyloidosis - clinical features, diagnosis and therapy. Aliment 18. Gilat T, Revach M, Sohar E. Deposition of amyloid in the
Pharmacol Ther. 2008;27(11):1006-1016. gastrointestinal tract. Gut. 1969;10(2):98-104.
3. Sipe JD, Benson MD, Buxbaum JN, Ikeda SI, Merlini G, 19. Alcalde-Vargas A, Leo-Carnerero E, Rojas-Mercedes N, Trigo-
Saraiva MJ, et al. Amyloid fibril proteins and amyloidosis: Salado C, Herrera-Justiniano JM, Márquez-Galán JL. Correlation
chemical identification and clinical classification International between location of amyloid deposits and endoscopic and clinical
Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. manifestations in symptomatic gastrointestinal amyloidosis. Rev
2016;23(4):209-213. Esp Enferm Dig. 2015;107(1):49-51.
4. Hazenberg BP. Amyloidosis: a clinical overview. Rheum Dis 20. Freudenthaler S, Hegenbart U, Schönland S, Behrens HM,
Clin North Am. 2013;39(2):323-345. Krüger S, Röcken C. Amyloid in biopsies of the gastrointestinal
5. Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva tract-a retrospective observational study on 542 patients. Virchows
MJ, et al. Nomenclature 2014: Amyloid fibril proteins and clinical Arch. 2016;468(5):569-577.
classification of the amyloidosis. Amyloid. 2014;21(4):221-224. 21. Hokama A, Kishimoto K, Nakamoto M, Kobashigawa C,
6. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Hirata T, Kinjo N, et al. Endoscopic and histopathological features
Engl J Med. 2003;349(6):583-596. of gastrointestinal amyloidosis. World J Gastrointest Endosc.
7. Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. 2011;3(8):157-161.
N Engl J Med. 1997;337(13):898-909. 22. Redondo-Cerezo E, Sánchez-Capilla AD, De La Torre-
8. Tada S, Iida M, Iwashita A, Matsui T, Fuchigami T, Yamamoto T, Rubio P, De Teresa J. Wireless capsule endoscopy: perspectives
et al. Endoscopic and biopsy findings of the upper digestive tract in beyond gastrointestinal bleeding. World J Gastroenterol.
patients with amyloidosis. Gastrointest Endosc. 1990;36(1):10-14. 2014;20(42):15664-15673.
9. Cowan AJ, Skinner M, Seldin DC, Berk JL, Lichtenstein 23. Yeh YC, Lin CH, Huang SC, Tsou YK. Education and
DR, O’Hara CJ, et al. Amyloidosis of the gastrointestinal tract: imaging. Gastrointestinal: gastric hematoma with bleeding in a
a 13-year, single-center, referral experience. Haematologica. patient with primary amyloidosis. J Gastroenterol Hepatol. 2014
2013;98(1):141-146. Mar; 29(3):419.
10. Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, 24. Kim SY, Moon SB, Lee SK, Hong SK, Kim YH, Chae
Hawkins PN, et al. Definition of organ involvement and treatment GB, et al. Light-chain amyloidosis presenting with rapidly
response in immunoglobulin light chain amyloidosis (AL): a progressive submucosal hemorrhage of the stomach. Asian J
consensus opinion from the 10th International Symposium on Surg. 2016;39(2):113-115.
Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am 25. Rosengren S, Mellqvist UH, Nahi H, Forsberg K, Lenhoff S,
J Hematol. 2005;79(4):319-328. Strömberg O, et al. Outcome of AL amyloidosis after high-dose
11. Hayman SR, Lacy MQ, Kyle RA, Gertz MA. Primary melphalan and autologous stem cell transplantation in Sweden,
systemic amyloidosis: a cause of malabsorption syndrome. Am J long-term results from all patients treated in 1994-2009. Bone
Med. 2001;111(7):535-540. Marrow Transplant. 2016;51(12):1569-1572.
12. Madsen LG, Gimsing P, Schiødt FV. Primary (AL) amyloidosis 26. Desport E, Bridoux F, Sirac C, Delbes S, Bender S, Fernandez
with gastrointestinal involvement. Scand J Gastroenterol. B, et al. Al amyloidosis. Orphanet J Rare Dis. 2012 Aug 21;7:54.
2009;44(6):708-711. doi: 10.1186/1750-1172-7-54.
13. Sonthalia N, Jain S, Pawar S, Zanwar V, Surude R, Rathi 27. Wilczek HE, Larsson M, Ericzon BG, FAPWTR. Long-term data
PM. Primary hepatic amyloidosis: A case report and review of from the Familial Amyloidotic Polyneuropathy World Transplant
literature. World J Hepatol. 2016;8(6):340-344. Registry (FAPWTR). Amyloid. 2011;18 Suppl 1:193-195.
14. Kaiserling E, Kröber S. Massive intestinal hemorrhage 28. Kumar SK, Gertz MA, Lacy MQ, Dingli D, Hayman SR,
associated with intestinal amyloidosis. An investigation Buadi FK, et al. Recent improvements in survival in primary
of underlying pathologic processes. Gen Diagn Pathol. systemic amyloidosis and the importance of an early mortality
1995;141(2):147-154. risk score. Mayo Clin Proc. 2011;86(1):12-18.
15. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal 29. Comenzo RL. Amyloidosis. Curr Treat Options Oncol.
Amyloidosis: Review of the Literature. Cureus. 2017 May 2006;7(3):225-236.
8;9(5):e1228. doi: 10.7759/cureus.1228.
Clujul Medical Vol. 91, No. 4, 2018: 469-473 473
You might also like
- 6 Neurology ModuleDocument46 pages6 Neurology ModuleCoffee MorningsNo ratings yet
- Word Power Made Easy SummaryDocument18 pagesWord Power Made Easy Summaryamitanand25tuNo ratings yet
- Henry's Clinical Diagnosis and Management by Laboratory Methods Twenty-Third Edition 2017Document20 pagesHenry's Clinical Diagnosis and Management by Laboratory Methods Twenty-Third Edition 2017Denise GuevaraNo ratings yet
- Granulomatous Appendicitis in A 12-Year-Old Boy: Derya Yayla, Bedriye Nuray Alpman, Yasemin DolekDocument3 pagesGranulomatous Appendicitis in A 12-Year-Old Boy: Derya Yayla, Bedriye Nuray Alpman, Yasemin Dolekansar ahmedNo ratings yet
- JGH 13992Document1 pageJGH 13992Dolly JazmiNo ratings yet
- Jurnal Volvulus InternaDocument6 pagesJurnal Volvulus InternaSiti Hardianty SugehaNo ratings yet
- en Hematochezia in Young Patient Due To CroDocument3 pagesen Hematochezia in Young Patient Due To Croseptian_tjayaNo ratings yet
- Jejunojejunal Intussusception As Initial Presentation of Coeliac Disease: A Case Report and Review of LiteratureDocument6 pagesJejunojejunal Intussusception As Initial Presentation of Coeliac Disease: A Case Report and Review of Literatureellya theresiaNo ratings yet
- Imaging Systemic Lupus Erythematosus Part 2Document11 pagesImaging Systemic Lupus Erythematosus Part 2menafabNo ratings yet
- Colonoscopy Induced Ischemic Colitis: An Endoscopic and Histological AssayDocument3 pagesColonoscopy Induced Ischemic Colitis: An Endoscopic and Histological AssayArfania LailyNo ratings yet
- Brunner's Gland Adenoma: Case ReportDocument3 pagesBrunner's Gland Adenoma: Case ReportasclepiuspdfsNo ratings yet
- 260-Article Text-875-1-10-20220728Document4 pages260-Article Text-875-1-10-20220728hasan andrianNo ratings yet
- Inflammatory Bowel Disease: Ruchita BhavsarDocument34 pagesInflammatory Bowel Disease: Ruchita BhavsarShinta MayasariNo ratings yet
- 2021 Article 2922Document4 pages2021 Article 2922AnirisulNo ratings yet
- Exogenic ReasonsDocument6 pagesExogenic ReasonsJohn wickNo ratings yet
- Laparoscopic Atypical Gastrectomy For Symptomatic Gastric LipomaDocument5 pagesLaparoscopic Atypical Gastrectomy For Symptomatic Gastric LipomaMaghfirah MahmuddinNo ratings yet
- Gastritis and Gastropathy Perspectives From The EndoscopistDocument4 pagesGastritis and Gastropathy Perspectives From The Endoscopisthenrique.uouNo ratings yet
- Cowden S Disease: A Case Report and Review of The LiteratureDocument4 pagesCowden S Disease: A Case Report and Review of The LiteratureKaisun TeoNo ratings yet
- Inflammatory Bowel Disease: - DR - Parashuram Professor Department of Internal Medicine DR Bramch, BengaluruDocument39 pagesInflammatory Bowel Disease: - DR - Parashuram Professor Department of Internal Medicine DR Bramch, BengaluruNeelesh PatilNo ratings yet
- 454 Jha A - 062018 PDFDocument3 pages454 Jha A - 062018 PDFVibhor Kumar JainNo ratings yet
- Gastro NotesDocument18 pagesGastro NoteslukeNo ratings yet
- Dr. Ali's Uworld Notes For Step 2 CK: GastroDocument32 pagesDr. Ali's Uworld Notes For Step 2 CK: GastrouyesNo ratings yet
- Colitis IndeterminadaDocument6 pagesColitis IndeterminadaMaria FannyNo ratings yet
- Comparison of Yogurt, Soybean, Casein, and Amino Acid-Based Diets in Children With Persistent DiarrheaDocument12 pagesComparison of Yogurt, Soybean, Casein, and Amino Acid-Based Diets in Children With Persistent DiarrheaFitriyana WinarnoNo ratings yet
- Ulcerative Colitis: An Update: Authors: Jonathan P SegalDocument5 pagesUlcerative Colitis: An Update: Authors: Jonathan P SegalManoel Victor Moreira MachadoNo ratings yet
- 40768-Article Text-143534-1-10-20190327Document3 pages40768-Article Text-143534-1-10-20190327nakibosmanNo ratings yet
- Approaches To Diagnosis Celiac DiseaseDocument16 pagesApproaches To Diagnosis Celiac DiseaseJunior GrobeNo ratings yet
- Eosinophilic Colitis: A Rare Case Report and Review of The LiteratureDocument8 pagesEosinophilic Colitis: A Rare Case Report and Review of The LiteratureIJAR JOURNALNo ratings yet
- Successful Outcome of Ineffective Infliximab Treated Autoimmune Enteropathy With Oral Budesonide 9800Document3 pagesSuccessful Outcome of Ineffective Infliximab Treated Autoimmune Enteropathy With Oral Budesonide 9800Desmo13No ratings yet
- "Belajar Mandiri 2.4.2" Penyakit Inslamasi Pada Saluran CernaDocument73 pages"Belajar Mandiri 2.4.2" Penyakit Inslamasi Pada Saluran CernaNununNo ratings yet
- Digestive Endoscopy - 2015 - Ohmiya - Obscure Gastrointestinal Bleeding Diagnosis and TreatmentDocument10 pagesDigestive Endoscopy - 2015 - Ohmiya - Obscure Gastrointestinal Bleeding Diagnosis and Treatmentpgomez156No ratings yet
- Kjim 2020 169Document2 pagesKjim 2020 169Houda El MoufidNo ratings yet
- Caz Bun 3Document7 pagesCaz Bun 3Dumitru RadulescuNo ratings yet
- Gastrite Enfisematosa - InglêsDocument7 pagesGastrite Enfisematosa - Inglêsamanda.gomesNo ratings yet
- GIT OSPE Pathology - Final-2 PDFDocument29 pagesGIT OSPE Pathology - Final-2 PDFafaq alismailiNo ratings yet
- Endoscopia: Diagnostic Approach of Intestinal Tuberculosis: Case and Literature ReviewDocument4 pagesEndoscopia: Diagnostic Approach of Intestinal Tuberculosis: Case and Literature ReviewDumitru RadulescuNo ratings yet
- By Subramaniyam Sabesan 4 Year Grodno State Medical UniversityDocument80 pagesBy Subramaniyam Sabesan 4 Year Grodno State Medical UniversitySam SabesNo ratings yet
- Gastritis Atrofica ReviewDocument33 pagesGastritis Atrofica ReviewFiorella Portella CordobaNo ratings yet
- 1471 230X 12 174 PDFDocument5 pages1471 230X 12 174 PDFAila HinlogNo ratings yet
- Askep IbdDocument43 pagesAskep IbdYou100% (1)
- Current Perspectives in Atrophic Gastritis: Pathogenesis and EpidemiologyDocument9 pagesCurrent Perspectives in Atrophic Gastritis: Pathogenesis and EpidemiologyFernando BorgesNo ratings yet
- Gastritis: Department of Gastroenterology General Hospital of Ningxia Medical University Si Cen MDDocument82 pagesGastritis: Department of Gastroenterology General Hospital of Ningxia Medical University Si Cen MDAvi Themessy100% (1)
- Inflammatory Bowel Disease (Ibd)Document28 pagesInflammatory Bowel Disease (Ibd)suhaNo ratings yet
- CSS Ulcerative ColitisDocument28 pagesCSS Ulcerative ColitisAthiyyah HasnaNo ratings yet
- Lesions Portal Gastritis Congestive Gastropathy?: Gastric in Hypertension: InflammatoryDocument7 pagesLesions Portal Gastritis Congestive Gastropathy?: Gastric in Hypertension: InflammatoryAnisa SafutriNo ratings yet
- Diseases of The Operated StomachDocument57 pagesDiseases of The Operated Stomachand3sgr3at100% (2)
- Hepatic Glycogenosis: An Under-Recognized Complication of Poorly Controlled Diabetes Mellitus Type 1 (Three Clinical Cases)Document6 pagesHepatic Glycogenosis: An Under-Recognized Complication of Poorly Controlled Diabetes Mellitus Type 1 (Three Clinical Cases)IJAR JOURNALNo ratings yet
- Coli TidesDocument11 pagesColi TidesAndrea Cayufilo CarmonaNo ratings yet
- Coeliac Disease: John S. Leeds, Andrew D. Hopper, and David S. SandersDocument14 pagesCoeliac Disease: John S. Leeds, Andrew D. Hopper, and David S. Sandersbdalcin5512No ratings yet
- Ulcerative Colitis: PseudopolypsDocument26 pagesUlcerative Colitis: PseudopolypsSocorro S. Gantalao-CorbedaNo ratings yet
- Inflammatoray Bowel DiseaseDocument14 pagesInflammatoray Bowel DiseaseSadr AkrmNo ratings yet
- Msn-Small Bowel Disorder - SeminarDocument71 pagesMsn-Small Bowel Disorder - SeminarSarithaRajeshNo ratings yet
- A Girl With Ulcerative Colitis in A Tertiary Care Hospital-A Case ReportDocument4 pagesA Girl With Ulcerative Colitis in A Tertiary Care Hospital-A Case ReportAbdullah KhanNo ratings yet
- A Girl With Ulcerative Colitis in A Tertiary Care Hospital-A Case ReportDocument4 pagesA Girl With Ulcerative Colitis in A Tertiary Care Hospital-A Case ReportWyn AgustinNo ratings yet
- Inflammatory Bowel DiseaseDocument15 pagesInflammatory Bowel DiseaseYanushka Bruce HerathNo ratings yet
- Michael John R. Aguilar, RMTDocument41 pagesMichael John R. Aguilar, RMTFrankenstein MelancholyNo ratings yet
- Endoscopic Hemostasis in Intensive Care Unit Patients With Upper Digestive Tract BleedingDocument7 pagesEndoscopic Hemostasis in Intensive Care Unit Patients With Upper Digestive Tract BleedingAgung KaryawinaraNo ratings yet
- Gupta 2008Document6 pagesGupta 2008Yacine Tarik AizelNo ratings yet
- IBD Final11Document41 pagesIBD Final11abraham debebeNo ratings yet
- IbdDocument67 pagesIbdchowhan04No ratings yet
- Gastric CarcinomaDocument43 pagesGastric CarcinomaPragadeswaran SNo ratings yet
- Body ImageDocument16 pagesBody Imageapi-518883731No ratings yet
- CYANOCOBALAMIN-cyanocobalamin Injection, S Olution Vitruvias TherapeuticsDocument12 pagesCYANOCOBALAMIN-cyanocobalamin Injection, S Olution Vitruvias TherapeuticsGreen HanauNo ratings yet
- Competencies For Nurses Working in Primary Health CareDocument16 pagesCompetencies For Nurses Working in Primary Health Carecarlos treichelNo ratings yet
- Amber Gemstone Can Amazingly Transform Negativity Into PositivityDocument3 pagesAmber Gemstone Can Amazingly Transform Negativity Into PositivityMustafa AliNo ratings yet
- Risk Assessment Record: Page 1 of 4Document4 pagesRisk Assessment Record: Page 1 of 4Abdul HadiNo ratings yet
- General Basis of Pathologic ConditionDocument24 pagesGeneral Basis of Pathologic Conditionallexa jimlaniNo ratings yet
- SARS-CoV-2 Infection in Acromegaly Patients Case Series-Poster Ese 2022Document2 pagesSARS-CoV-2 Infection in Acromegaly Patients Case Series-Poster Ese 2022Ank BaiceanuNo ratings yet
- Acid Base BalanceDocument44 pagesAcid Base BalanceKenny JapNo ratings yet
- 30 Poultry Diseases Online Course Module 1 of 3Document9 pages30 Poultry Diseases Online Course Module 1 of 3daniel mundawaroNo ratings yet
- Understanding Tick Bites and Lyme DiseaseDocument2 pagesUnderstanding Tick Bites and Lyme DiseasejeffNo ratings yet
- PneumonoultramicroscopicsilicovolcanoconiosisDocument1 pagePneumonoultramicroscopicsilicovolcanoconiosisdaria popescuNo ratings yet
- Definition, Etiology, Epidemiology PepticDocument4 pagesDefinition, Etiology, Epidemiology Pepticalfira andiniNo ratings yet
- Turley5e CH03 PPT AccessibleDocument103 pagesTurley5e CH03 PPT Accessiblekareen.arciaga1981No ratings yet
- Tut 202 2018 S1Document23 pagesTut 202 2018 S1Feroza AngamiaNo ratings yet
- Pathologycal PhysiologyDocument29 pagesPathologycal PhysiologyArsilan Aziz LoneNo ratings yet
- Causes of Ischemic Stroke: October 2010Document19 pagesCauses of Ischemic Stroke: October 2010Pajokka 10No ratings yet
- Q3 - LESSON PLAN IN HEALTH8 Cot Final NEW PPST STANDARDDocument9 pagesQ3 - LESSON PLAN IN HEALTH8 Cot Final NEW PPST STANDARDjuvelyn abuganNo ratings yet
- Favipiravir Clinical Vs ArbidolDocument30 pagesFavipiravir Clinical Vs Arbidoladel ehabNo ratings yet
- Lobar Function Test.Document57 pagesLobar Function Test.DEEPA NAIRNo ratings yet
- Clerking Sheet 1Document8 pagesClerking Sheet 1Precious ChaiNo ratings yet
- Lepy 103Document19 pagesLepy 103Syed FerozNo ratings yet
- CHAPTER 5 InflammationDocument51 pagesCHAPTER 5 Inflammationyeshita yonasNo ratings yet
- Organon Visuum: Tri Indah Winarni, MD, PHD Anatomy Dept. Faculty of Medicine Diponegoro UniversityDocument46 pagesOrganon Visuum: Tri Indah Winarni, MD, PHD Anatomy Dept. Faculty of Medicine Diponegoro UniversityLailatuz ZakiyahNo ratings yet
- Health MateDocument3 pagesHealth MateAnisah AquilaNo ratings yet
- Otitis Externa: Straight To The Point of CareDocument45 pagesOtitis Externa: Straight To The Point of CareNana OkujavaNo ratings yet
- Juvenile Idiopathic Arthritis Case StudyDocument4 pagesJuvenile Idiopathic Arthritis Case StudyBruno PereiraNo ratings yet
- Zoladex 3.6mg Implant - Summary of Product Characteristics (SMPC) - Print Friendly - (Emc)Document8 pagesZoladex 3.6mg Implant - Summary of Product Characteristics (SMPC) - Print Friendly - (Emc)KunalNo ratings yet
- The Content of This Leaflet Was Updated According To The Guidelines of The Ministry of Health in September 2017Document14 pagesThe Content of This Leaflet Was Updated According To The Guidelines of The Ministry of Health in September 2017ddandan_2No ratings yet