Professional Documents
Culture Documents
CHROMATOGRAPHY
Uploaded by
ᴇᴜᴘʜᴏʀɪxOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHROMATOGRAPHY
Uploaded by
ᴇᴜᴘʜᴏʀɪxCopyright:
Available Formats
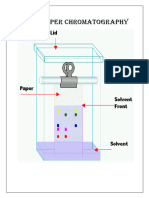
EXPERIMENT NO : DATE:
AIM -To separate the colour components present in the mixture of inks by
ascending paper chromatography and find their Rf values .
APPARATUS REQUIRED : Gas jar ,Glass rod ,Whatman’s Filter paper ,Jar cover ,Fine capillary tube
Chemicals Used : Mixtrue of colour or black sketch pen, Distilled water
Theory :
In paper chromatography, water molecules present in the pores of the filter paper
act as the stationary phase and the moving phase can be a solvent like water .
As the moving phase passes through the spot on which sample has been adsorbed,
it dissolves the components more or less readily; depending upon the solubility and
carries them along with it while moving on the support. At a given temperature and
for a given solvent, it is possible to determine the characteristic rate of movement of
each substance on the chromotographic paper, as the moving phase moves. This is
represented by relative front or retardation factor also
called Rf value. Rf values of different compounds are different even if the mobile
phase (solvent) is same.
Since solvent front moves faster than the compounds, the Rf value of a substance
will always be less than one. Also note that Rf value has no unit.
OBSERVATION AND CALCULATION
S.No Substance Distance Distance Rf value
travelled by travelled by
coloured solvent
component
Mixture of ink
Mixture of ink
Result
(i) Rf values of components __________.
(ii) Rf values of components
(a) Dip the paper strip in the solvent in such a way that the spot of the mixture is
above the solvent level and the movement of the solvent front is not zig-zag.
(b) While spotting the test solution on the paper, do not allow the spots to spread.
c)Use finely drawn capillary to put the spot on the paper.
(d) Ensure that the filter paper strip hangs freely in the jar.
(e) Once the experiment is set, do not disturb the jar as long as the chromatogram is
being developed.
NOTE Paste the chromatography paper on left side paper
You might also like
- Experiment 1 ChromatographyDocument2 pagesExperiment 1 ChromatographyNandita KrishnanNo ratings yet
- ChromatographyDocument3 pagesChromatographyAashiNo ratings yet
- CHEMDocument13 pagesCHEMMohamed MusthapaNo ratings yet
- 37 Analytical Techniques PDFDocument225 pages37 Analytical Techniques PDFKomal EhsanNo ratings yet
- Chromatography Student 2021-2022Document17 pagesChromatography Student 2021-2022Nickoye WilliamsNo ratings yet
- ChromatographyDocument4 pagesChromatographyDavies MasumbaNo ratings yet
- Chromatography and Paper ChromatographyDocument29 pagesChromatography and Paper Chromatographymostafa33puvmNo ratings yet
- Paper Chromatography Model and Practical LabDocument4 pagesPaper Chromatography Model and Practical Labdebbie bongNo ratings yet
- Chromatography ReportDocument10 pagesChromatography Reportwei linNo ratings yet
- Paper Chromatography 2Document4 pagesPaper Chromatography 2Emily MangraNo ratings yet
- 2: Paper Chromatography of Gel Ink Pens (Experiment) : ObjectivesDocument3 pages2: Paper Chromatography of Gel Ink Pens (Experiment) : ObjectivesDivya TripathyNo ratings yet
- TLC Demonstration Separating MixturesDocument7 pagesTLC Demonstration Separating MixturesNanditha ANo ratings yet
- Air Force School Ambala CanttDocument16 pagesAir Force School Ambala Canttsimran ranaNo ratings yet
- Ipchmclass12th Converted1 191208134113Document15 pagesIpchmclass12th Converted1 191208134113rathorepadamsingh698No ratings yet
- CHROMATOGRAPHIC TECHNIQUES 1200328060603035050Document12 pagesCHROMATOGRAPHIC TECHNIQUES 1200328060603035050KIRAN ALLUNo ratings yet
- 3.16 Revision Guide Chromatography AqaDocument3 pages3.16 Revision Guide Chromatography AqaRS JNo ratings yet
- AlexDocument2 pagesAlexYasar Arafath SNo ratings yet
- Retention FactorDocument1 pageRetention Factorsirrah jonesNo ratings yet
- DocumentDocument21 pagesDocumentcᴘcтԍᴀмιɴԍ YTNo ratings yet
- S.Y B. Sc. AC – 202 Unit III CHROMATOGRAPHYDocument18 pagesS.Y B. Sc. AC – 202 Unit III CHROMATOGRAPHYYerram Raju BeharaNo ratings yet
- ChromatographyDocument6 pagesChromatographyUmer AyazNo ratings yet
- Paper ChromatographyDocument17 pagesPaper ChromatographyWhy I am not VIRAT KOHLINo ratings yet
- Lab 3 - Separation of PH Indicators Using Paper ChromatographyDocument6 pagesLab 3 - Separation of PH Indicators Using Paper ChromatographyJesiann SmithNo ratings yet
- Analytical Techniques Final Note NADocument121 pagesAnalytical Techniques Final Note NAyuvi78312No ratings yet
- Che ChromatographyDocument15 pagesChe ChromatographyArpit MauryaNo ratings yet
- Unit 5Document69 pagesUnit 5NTGNo ratings yet
- 6.13 Analytical TechniquesDocument12 pages6.13 Analytical TechniquesPedro Moreno de SouzaNo ratings yet
- ChromatographyDocument25 pagesChromatographyMani JeeNo ratings yet
- Lab Report Exp 6Document5 pagesLab Report Exp 6api-384913960No ratings yet
- Lesson 1 IntroductionDocument17 pagesLesson 1 IntroductionGemma Wrigley100% (1)
- ChromatographyDocument6 pagesChromatographyAyush K. SharmaNo ratings yet
- HrmtograhiDocument8 pagesHrmtograhiALJOREY LAZARITONo ratings yet
- Separate chemicals with paper chromatographyDocument4 pagesSeparate chemicals with paper chromatographyMooma fatimaNo ratings yet
- Identify Unknown Compounds with Paper ChromatographyDocument6 pagesIdentify Unknown Compounds with Paper ChromatographyPol Marasigan BanzonNo ratings yet
- Chem ChromatographyDocument15 pagesChem ChromatographyArpit MauryaNo ratings yet
- Principle of Paper Chromatography: The Separation in Paper Chromatography Is AchievedDocument12 pagesPrinciple of Paper Chromatography: The Separation in Paper Chromatography Is AchievedSoumik MahapatroNo ratings yet
- Paper ChromatographyDocument5 pagesPaper ChromatographyMaria Elena PascualNo ratings yet
- Bioc 211Document6 pagesBioc 211Femina ArgonzaNo ratings yet
- Chemistry Project Paper ChromatographyDocument20 pagesChemistry Project Paper ChromatographyAmrita SNo ratings yet
- ChromaaaaaalateDocument7 pagesChromaaaaaalateVanessaOlgaJ.DagondonNo ratings yet
- ChromatographyDocument40 pagesChromatographySAGAR POUDELNo ratings yet
- Separate Colored Ink Components Using Paper ChromatographyDocument2 pagesSeparate Colored Ink Components Using Paper ChromatographywibowosukandiNo ratings yet
- Experiment 2 ChromatographyDocument4 pagesExperiment 2 ChromatographySuryansh MalikNo ratings yet
- ChromatographyDocument13 pagesChromatographysujeetkverma.7250No ratings yet
- SEMESTER 3 Practical Science 2 Experiment 8 TopicDocument6 pagesSEMESTER 3 Practical Science 2 Experiment 8 Topickat tunNo ratings yet
- Paper ChromatographyDocument5 pagesPaper ChromatographyDr. P.S.SenguptaNo ratings yet
- B.tech. Biotechnology NotesDocument2 pagesB.tech. Biotechnology NotesMudit MisraNo ratings yet
- Samriddhi Chromatography ProjectDocument17 pagesSamriddhi Chromatography Projectcᴘcтԍᴀмιɴԍ YTNo ratings yet
- Lab Report TLCDocument6 pagesLab Report TLCkashvinwarmaNo ratings yet
- BEP1021 - Group 3 Experiment 4Document16 pagesBEP1021 - Group 3 Experiment 4Tasmea sultanaNo ratings yet
- ChromatographyDocument56 pagesChromatographyapi-26998277100% (14)
- Unit 5 ChromatographyDocument86 pagesUnit 5 ChromatographyRujal KundhareNo ratings yet
- C3. TLCDocument26 pagesC3. TLCgheevarghesedevasiaNo ratings yet
- Chemistry ProjectDocument28 pagesChemistry ProjectVikramjeet Singh82% (11)
- Experiment 2 PharmacognosyDocument2 pagesExperiment 2 PharmacognosyAbdullah YousafzaiNo ratings yet
- Chemistry Lab 1 Paper ChromatographyDocument5 pagesChemistry Lab 1 Paper ChromatographyJhourshaiqrylle Wynch LozadaNo ratings yet
- Experiment 2 ChromatographyDocument6 pagesExperiment 2 ChromatographyFranz CandidoNo ratings yet
- Stochastic Methods for Flow in Porous Media: Coping with UncertaintiesFrom EverandStochastic Methods for Flow in Porous Media: Coping with UncertaintiesNo ratings yet