Professional Documents
Culture Documents
Chemy Expt 4
Uploaded by
Engr Saud shahOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemy Expt 4
Uploaded by
Engr Saud shahCopyright:
Available Formats
University of Bahrain
Collage of science
Department of chemistry
Experiment4
Preparation of cyclohexane, and tests for unsaturation.
Name:- Hajer abdulrahman Hussain
Student ID:-20144134
Lab Instructor:- Dr.rema
Objectives:-
Dehydration of cyclohexanol to get cyclohexene and identifying
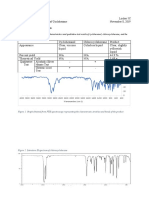
the product to by using Gas Chromatography (GC) and performing
tests of unsaturation.
Introduction:-
Cyclohexane is a cycloalkane with the molecular formula C6H12.
Cyclohexane is mainly used for the industrial production of adipic
acid and caprolactam, which are precursors to nylon. Cyclohexane
is a colorless, flammable liquid with a distinctive detergent-like
odor, reminiscent of cleaning products (in which it is sometimes
used.
In organic chemistry, the bromine test is a qualitative test for the
presence of unsaturation (carbon to carbon double or triple bonds)
and phenols.
Data & calculation:-
Cyclohexanol:
Grams used = 8 g
Molecular weight= 100.16 g/mol
Moles used = mass/ molecular weight
= 8 / 100.16 = 0.0799 moles
Cyclohexene:
Molecular weight = 82.14 g/mol
Moles expected = 0.0799 moles
Grams expected : 100.16 g → 82.14 g
8g → X
X = 6.561 g (Theoretical yield)
Weight of empty flask= 26.406 g
Weight of flask with filtrate = 29.922 g
Hence, Grams obtained: (Actual yield) = 3.516 g
Percentage yield = (actual yield / theoretical yield) * 100
= (3.516 / 6.561) * 100 = 53.58 %
Conclusion:-
We have correctly identified our product as cyclohexene which we have
obtained from the dehydration of cyclohexanol . Identification was obtained
by performing Gas Chromatography and also tests of unsaturation.
You might also like
- Methods and Instruments Used in Brewing Control - Selected QuestionsFrom EverandMethods and Instruments Used in Brewing Control - Selected QuestionsNo ratings yet
- Experiment #1Document7 pagesExperiment #1Lakani Tindiwi YangalaNo ratings yet
- A Laboratory Manual of Physical PharmaceuticsFrom EverandA Laboratory Manual of Physical PharmaceuticsRating: 2.5 out of 5 stars2.5/5 (2)
- Chm457 Fundamental Organic Chemistry: Experiment 2: Preparation of 4 - MethylcyclohexeneDocument11 pagesChm457 Fundamental Organic Chemistry: Experiment 2: Preparation of 4 - MethylcyclohexeneNur HismanizaNo ratings yet
- Practical Manual of Analytical ChemistryFrom EverandPractical Manual of Analytical ChemistryRating: 4.5 out of 5 stars4.5/5 (3)
- The Aldol Condensation Reaction - Preparation of Benzalacetophenones (Chalcones)Document6 pagesThe Aldol Condensation Reaction - Preparation of Benzalacetophenones (Chalcones)Amirul Azhar93% (30)
- Lab Report C3Document5 pagesLab Report C3SumayyahNo ratings yet
- CHM557 Exp 2Document12 pagesCHM557 Exp 2syafNo ratings yet
- Exp10 PDFDocument3 pagesExp10 PDFعمر العنزيNo ratings yet
- 18551507-019 CHEM-312 Lab ManualDocument8 pages18551507-019 CHEM-312 Lab ManualRukhsana Malik100% (1)
- CHM 556 Organic Chemistry 2 Experiment 2: Reduction of CyclohexanoneDocument5 pagesCHM 556 Organic Chemistry 2 Experiment 2: Reduction of CyclohexanoneAmirul Azhar100% (1)
- Food Chemistry PracticalDocument7 pagesFood Chemistry PracticalSamip PandeyNo ratings yet
- Lab Report Exp. 4 CHM457 Fund. Organic ChemistryDocument7 pagesLab Report Exp. 4 CHM457 Fund. Organic ChemistryHusnul HakimNo ratings yet
- CamphorDocument7 pagesCamphorashNo ratings yet
- Organic Experiment 7Document7 pagesOrganic Experiment 7Abdulaziz KaramiNo ratings yet
- Laboratort Report of Introduction To Organic Chemistry (CHM 413)Document5 pagesLaboratort Report of Introduction To Organic Chemistry (CHM 413)biokimia 2018No ratings yet
- Final Project Chem BamDocument3 pagesFinal Project Chem Bamapi-338950517No ratings yet
- CHM 457 Exp 2Document9 pagesCHM 457 Exp 2Ayish MataNo ratings yet
- Carbonyl Ex 3Document5 pagesCarbonyl Ex 3Arabella VirgoNo ratings yet
- Aldol Condensation ReactionDocument8 pagesAldol Condensation ReactionMohd Nakirudin Muhamad Nor100% (1)
- Last Post Labs (7 and 8)Document6 pagesLast Post Labs (7 and 8)Chell AtaizaNo ratings yet
- Preparation of ChlorocyclohexaneDocument5 pagesPreparation of ChlorocyclohexaneMarjory CastilloNo ratings yet
- Experiment 2 Preparation of 4 Methylcyclohexene From Dehydration of 4 MethylcyclohexanolDocument7 pagesExperiment 2 Preparation of 4 Methylcyclohexene From Dehydration of 4 MethylcyclohexanolHusnul HakimNo ratings yet
- Acid-Catalyzed Dehydration of Cyclohexanol To Cyclohexene Lab - ReportDocument7 pagesAcid-Catalyzed Dehydration of Cyclohexanol To Cyclohexene Lab - ReportMcAdam TULAPI100% (1)
- Chm457 Experiment 2Document7 pagesChm457 Experiment 2sofeaNo ratings yet
- Preparation of 4-Methylcyclohexene From Dehydration of 4-MethylcyclohexanolDocument8 pagesPreparation of 4-Methylcyclohexene From Dehydration of 4-MethylcyclohexanolHidayu AdnanNo ratings yet
- E6Document7 pagesE6Leon LaiNo ratings yet
- Organic Chemistry Laboratory: Report 8: Fischer Ester SynthesisDocument7 pagesOrganic Chemistry Laboratory: Report 8: Fischer Ester SynthesisPhú NguyễnNo ratings yet
- Pac 213 Prac 2Document6 pagesPac 213 Prac 2Tlotliso MphomelaNo ratings yet
- 95% ETANOL SOXHLETbbhjhhjghDocument5 pages95% ETANOL SOXHLETbbhjhhjghRani RubiyantiNo ratings yet
- Experiment 8 Preparation of Cyclohexene From CyclohexanolDocument6 pagesExperiment 8 Preparation of Cyclohexene From CyclohexanolAishah Cnd100% (1)
- FAR 113-Prac 3Document9 pagesFAR 113-Prac 3Arvin KumarNo ratings yet
- Lab Report ExtractionDocument6 pagesLab Report Extractionnicolas reyNo ratings yet
- CHM557 Experiment 4 - The Aldol Condensation Reaction - Preparation of Benzalacerophenones (Chalcones)Document7 pagesCHM557 Experiment 4 - The Aldol Condensation Reaction - Preparation of Benzalacerophenones (Chalcones)MamamiaNo ratings yet
- Sodium Borohydride Reduction of Cyclohex PDFDocument8 pagesSodium Borohydride Reduction of Cyclohex PDFhahadindongNo ratings yet
- Chlorophyll - MSC ProjectDocument4 pagesChlorophyll - MSC ProjectPradeepNo ratings yet
- Lab Report Experiment 2 CHM457Document9 pagesLab Report Experiment 2 CHM457lala lalaNo ratings yet
- Research Article: Isolation and Characterization of Antitumor Alkaloid From Poppy Capsules (Document5 pagesResearch Article: Isolation and Characterization of Antitumor Alkaloid From Poppy Capsules (Muhammad Ihda HlzNo ratings yet
- Exp5 latestTTTDocument8 pagesExp5 latestTTTzarif luqmaniNo ratings yet
- Post Lab 2Document9 pagesPost Lab 2Chin RamosNo ratings yet
- Método Colorimetrico para Determinação de ColagenoDocument4 pagesMétodo Colorimetrico para Determinação de ColagenoRoberta RochaNo ratings yet
- Robinson Annulation Reaction of NItrochalconeDocument10 pagesRobinson Annulation Reaction of NItrochalconeMohd Nakirudin Muhamad NorNo ratings yet
- Analysis of Elimination Reaction of CyclohexanolDocument4 pagesAnalysis of Elimination Reaction of CyclohexanolPratiwi Surya RahayuNo ratings yet
- Soduim Borohydride Reduction of CyclohexanoneDocument10 pagesSoduim Borohydride Reduction of CyclohexanoneHawra JawadNo ratings yet
- Experiment S5 - Isolation of Eugenol From Cloves - 18387918Document6 pagesExperiment S5 - Isolation of Eugenol From Cloves - 18387918stevefox0860% (1)
- Siti Nur Afiqah Binti Mahazan - As2533a1 - Exp 2Document10 pagesSiti Nur Afiqah Binti Mahazan - As2533a1 - Exp 2SITI NUR AFIQAH MAHAZANNo ratings yet
- Synthesis of Cyclohexene Lab ReportDocument3 pagesSynthesis of Cyclohexene Lab ReportMARIANA ÁLVAREZ GÓMEZNo ratings yet
- Exp Nana 1Document7 pagesExp Nana 1ellymanisNo ratings yet
- Lab 2 - Preparation of AcetaminophenDocument4 pagesLab 2 - Preparation of Acetaminophenapi-281862068100% (3)
- Experiment 2 457Document7 pagesExperiment 2 457Sarah HannisNo ratings yet
- ObjectiveDocument8 pagesObjectivenaim rashidNo ratings yet
- Sulfonation of Aniline ExperimentDocument3 pagesSulfonation of Aniline Experimentpakpolitics206No ratings yet
- IndolinoneDocument93 pagesIndolinonePranav ParmarNo ratings yet
- NaBH4 Reduction of CyclohaxanoneDocument5 pagesNaBH4 Reduction of Cyclohaxanonenurul1110No ratings yet
- Experiment 7 Analysis of Chlorpyrifos in Water by Solid-Phase Extraction (SPE) and Gas Chromatography - Electron Captured Detector (ECD)Document6 pagesExperiment 7 Analysis of Chlorpyrifos in Water by Solid-Phase Extraction (SPE) and Gas Chromatography - Electron Captured Detector (ECD)Maxvicklye RaynerNo ratings yet
- Experiment 2 chm556 Organic ChemistryDocument8 pagesExperiment 2 chm556 Organic ChemistryAmar SafwanNo ratings yet
- Purification of Acetanilide Via RecrystallizationDocument10 pagesPurification of Acetanilide Via RecrystallizationLouisiana Sollestre0% (1)
- Deamination Lab ReportDocument4 pagesDeamination Lab ReportRyanJForteNo ratings yet
- Exp 4 chm556Document7 pagesExp 4 chm556Azli AzmanNo ratings yet
- 6508 Assignment 2Document15 pages6508 Assignment 2Engr Saud shahNo ratings yet
- CHALLANDocument1 pageCHALLANEngr Saud shahNo ratings yet
- 1 s2.0 S0016236117301047 MainDocument9 pages1 s2.0 S0016236117301047 MainEngr Saud shahNo ratings yet
- Reservoir SimulationDocument15 pagesReservoir SimulationEngr Saud shahNo ratings yet
- Lecture 18,19 - Water Pollution - IIDocument25 pagesLecture 18,19 - Water Pollution - IIEngr Saud shahNo ratings yet
- Lecture 15,16,17 - Water Pollution-IDocument52 pagesLecture 15,16,17 - Water Pollution-IEngr Saud shahNo ratings yet
- Chapter 2 Page 5Document1 pageChapter 2 Page 5Engr Saud shahNo ratings yet
- Chapter 2 Page 6Document1 pageChapter 2 Page 6Engr Saud shahNo ratings yet
- ICH Quality Guidelines: An Implementation GuideFrom EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdaleNo ratings yet
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- It's Elemental: The Hidden Chemistry in EverythingFrom EverandIt's Elemental: The Hidden Chemistry in EverythingRating: 4 out of 5 stars4/5 (10)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeFrom EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeRating: 5 out of 5 stars5/5 (1)
- Guidelines for Defining Process Safety Competency RequirementsFrom EverandGuidelines for Defining Process Safety Competency RequirementsRating: 3 out of 5 stars3/5 (1)
- The Production of Volatile Oils and Perfumery Plants in the United StatesFrom EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesNo ratings yet
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeFrom EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Taste: Surprising Stories and Science About Why Food Tastes GoodFrom EverandTaste: Surprising Stories and Science About Why Food Tastes GoodRating: 3 out of 5 stars3/5 (20)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (1)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- The Billion-Dollar Molecule: The Quest for the Perfect DrugFrom EverandThe Billion-Dollar Molecule: The Quest for the Perfect DrugRating: 5 out of 5 stars5/5 (2)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesFrom EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesRating: 5 out of 5 stars5/5 (2)