Professional Documents
Culture Documents
Y12 April Assessment ANS
Y12 April Assessment ANS
Uploaded by
Anela XVIIOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Y12 April Assessment ANS
Y12 April Assessment ANS
Uploaded by
Anela XVIICopyright:
Available Formats
Worthing College Page 1 of 8
Mark schemes
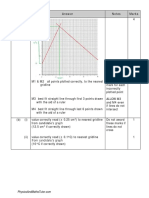
(a)
1
M1 Curve is higher and displaced to the left
M2 Only crosses the original curve once
2
(b) Rate of reaction decreases (no mark)
Fewer particles will have energy greater than or equal to the activation energy
1
Fewer successful collisions in a given time
Less frequent successful collisions
1
(c) The amount of gas present (or number of molecules) has been reduced / or the pressure
has been reduced
1
Rate of reaction decreases (no mark)
Particles are spread further apart
1
Fewer collisions between gas particles so fewer successful collisions
1
[7]
Worthing College Page 2 of 8
(a) Method 1
2
Allow working throughout to 2sf
M1 Moles of Mg = 0.396/24.3 = 0.0163
1
M2 Moles of CH3COOH = 0.600 × 30.0/1000 = 0.018
1
M3 Mark for showing Mg is in excess: either
0.018 mol of CH3COOH reacts with 0.009 mol of Mg OR
0.0163 mol of Mg reacts with 0.0326 mol of CH3COOH OR
0.0073 mol of Mg is in excess
1
If candidate gets 16.3 mol (as not converted mg to g) in method 1 or
3 then can only score 1 mark maximum (M2)
Accept other valid calculations that show the Mg is in excess
Method 2
M1 Moles of CH3COOH = 0.600 × 30.0/1000 = 0.018
M2 Moles of Mg that would react with this = 0.009
M3 Mass of Mg needed = 24.3 × 0.009 = 0.219 g which is less
than 0.396 g OR
Moles of Mg = 0.0163 which is more than 0.009 required
Method 3
M1 Moles of Mg = 0.396/24.3 = 0.0163
M2 Moles of CH3COOH that would react with this = 0.0326
M3 Volume of CH3COOH needed = 0.0326 / 0.60 = 0.0543 dm3
(54.3 cm3) which is more than 0.030 dm3 (30 cm3)
(b) M1 Line starts at origin and is steeper
1
M2 (moles CH3COOH = 0.800 × 20/1000 = 0.016) line levels out
on 8th line up (line below the original 9th line)
M2 for line on 8th line on grid (original on 9th line) – allow some
leniency so long as clear it ends at (or very close to) the 8th line;
and line does not significantly wobble
1
[5]
Worthing College Page 3 of 8
(a) (i) M1
3 High (temperature) OR Increase (the temperature)
If M1 is incorrect CE = 0 for the clip
If M1 is blank, mark on and seek to credit the correct information
in the text
M2
The (forward) reaction / to the right is endothermic or takes in / absorbs heat
OR
The reverse reaction / to the left is exothermic or gives out / releases heat
M3 depends on correct M2 and must refer to temperature / heat
M3 depends on a correct statement for M2
At high temperature, the (position of ) equilibrium shifts / moves left to right to
oppose the increase in temperature
For M3, the position of equilibrium shifts / moves
to absorb heat OR
to lower the temperature OR
to cool down the reaction
3
(ii) M1
The reaction gets to equilibrium faster / in less time
OR
Produces a small yield faster / in less time
OR
Increases the rate (of reaction / of attainment of equilibrium)
Mark independently
M2
High pressure leads to one of the following
• more particles / molecules in a given volume
• particles / they are closer together
• higher concentration of particles / molecules
AND
• more collisions in a given time / increased collision frequency
Penalise M2 for reference to increased energy of the particles
2
(iii) M1 Increase in / more / large(r) / big(ger) surface area / surface sites
Mark independently
For M1 accept “an increase in surface”
M2 increase in / more successful / productive / effective collisions (in a given
time) (on the surface of the catalyst / with the nickel)
For M2 not simply “more collisions”
Ignore “the chance or likelihood” of collisions
2
Worthing College Page 4 of 8
(b) M1
No effect / None
If M1 is incorrect CE = 0 for the clip
If M1 is blank, mark on and seek to credit the correct information
in the text
M2 requires a correct M1
Equal / same number / amount of moles / molecules / particles on either side of the
equation
OR
2 moles / molecules / particles on the left and 2 moles / molecules / particles on the
right
M2 depends on a correct statement for M1
In M2 not “atoms”
2
[9]
(a) (i) (Kp) = (pz)2/(px)(py)3
4
(penalise use of square brackets, allow ())
1
(ii) X (22–6)/4 = 4 (MPa)
(mark is for value 4 only, ignore units)
1
Y obtained by multiplying value for X by 3
(allow conseq on wrong value for X)
1
Y 4.0 × 3 = 12 (MPa)
(mark is for value 12 only)
1
(iii) Kp = 6.02/4.0 × 12.03 = 5.21 × 10–3
(allow conseq on wrong values for X and
Y e.g.62/3 × 93 = 0.165)
(if Kp wrong in (a)(i) CE)
1
MPa–2
(allow any unit of P–2 provided ties to P used for Kp value)
1
(b) high pressure expensive (due to energy or plant costs)
1
(Rate is) slow (at lower temperatures)
1
[8]
Worthing College Page 5 of 8
(a) mol R = 2x
5 1
(b) 3.6 =
M1 can be awarded for the insertion of their answer from (a)
correctly
1
√3.6 = (only positive root to be used)
M2 can be awarded if their expression is expanded
1
√3.6 −√3.6 x = 2x
1.9 = 3.9x
X = 0.49
[R] = 0.97 mol dm−3 (allow range 0.97–.098)
M3 solve for x from their expression in M1 and use it to calculate [R]
1
[4]
6
(a) Kc =
Penalise ( ) in this part but can score units; mark on in (b)
If Kc expression wrong no marks in this part but can score M1 & M3
in (b)
1
units = mol−1 dm3
1
Worthing College Page 6 of 8
(b) [O2] = or or
Correct answer scores three marks
Ignore ( ) in this part
Penalise contradiction in M1
M1
1
0.061(4)
If Kc expression wrong in (a) can score M1 here for rearrangement
of their Kc & M3 for multiplication by 1.4
M2
mol O2 = 0.0614 × 1.4 = 0.086 (allow 0.085−0.087)
If Kc or rearrangement wrong here score only M3 for multiplication
by 1.4
1
M3 = correct answer of (M2 × 1.4)
M3
1
(c) (i) No effect OR none OR no change OR stays the same
1
(ii) Effect: Increase or more SO3
Increase or more SO3
If wrong effect, no further marks, but M2 and
M3 are independent of each other
M1
1
Fewer mole(cule)s on RHS
or 3 moles to 2 moles
or (eqm shifts) to side with fewer moles
(V3 or) residual V decreases in numerator of Kc expression
M2
1
Worthing College Page 7 of 8
Equilibrium moves / shifts to reduce the pressure /
oppose the increase in pressure
to keep Kc constant,
ratio
must increase
Allow to oppose the change only if increase pressure mentioned
M3
1
[9]
Worthing College Page 8 of 8
You might also like
- Unofficial Mark SchemeDocument6 pagesUnofficial Mark SchemeRodolph SmithNo ratings yet
- Chemistry Paper 2 TZ2 HL MarkschemeDocument18 pagesChemistry Paper 2 TZ2 HL MarkschemeMehek AhujaNo ratings yet
- Mark Scheme (Results) January 2020: Pearson Edexcel International GCSE in Chemistry (4CH1) Paper 1CDocument23 pagesMark Scheme (Results) January 2020: Pearson Edexcel International GCSE in Chemistry (4CH1) Paper 1Cmostafa barakat85% (13)
- Mass Spec Exam Questions 1Document7 pagesMass Spec Exam Questions 1booboo100% (1)
- Chemsheets A2 1001 Kinetics BookletDocument20 pagesChemsheets A2 1001 Kinetics BookletMarc WilfordNo ratings yet
- Vacuum Engineering Calculations, Formulas, and Solved ExercisesFrom EverandVacuum Engineering Calculations, Formulas, and Solved ExercisesRating: 4.5 out of 5 stars4.5/5 (2)
- BASF Oase Gas-TreatmentDocument12 pagesBASF Oase Gas-Treatmentheri0% (1)
- TML ProcedureDocument19 pagesTML Procedurejeswin100% (1)
- Completion EquipmentsDocument16 pagesCompletion EquipmentsAngel Ngo100% (3)
- Mrofso: Number Answer Notes MarksDocument14 pagesMrofso: Number Answer Notes MarksSalmuel SmithNo ratings yet
- Chemical Formulae, Equations, Calculations 2 MSDocument8 pagesChemical Formulae, Equations, Calculations 2 MSStudy EmailNo ratings yet
- Rates of Reaction 3 MSDocument11 pagesRates of Reaction 3 MSSadika BintaNo ratings yet
- Jan 2020 1CDocument23 pagesJan 2020 1CssonersarsilmazzNo ratings yet
- The Periodic Table MSDocument15 pagesThe Periodic Table MSAli SayedNo ratings yet
- Rates of Reaction 3 MSDocument13 pagesRates of Reaction 3 MSabhannanNo ratings yet
- Rates of Reaction 2 MSDocument9 pagesRates of Reaction 2 MSSadika BintaNo ratings yet
- 9701 m16 Ms 52 PDFDocument4 pages9701 m16 Ms 52 PDFZAHRA HUSSAIN(Student)No ratings yet
- 7,8,9 (MS)Document26 pages7,8,9 (MS)devil's queenNo ratings yet
- 6.1 Ionic Bonding (1C) MSDocument10 pages6.1 Ionic Bonding (1C) MSsonnyNo ratings yet
- Acids, Alkalis and Titrations 1 MSDocument10 pagesAcids, Alkalis and Titrations 1 MSNewton JohnNo ratings yet
- Organic Analysis MS 2Document19 pagesOrganic Analysis MS 2Hamza AbulailaNo ratings yet
- Met304 SchemeDocument30 pagesMet304 SchemenikhilasoknNo ratings yet
- Energetics Exam Q Booklet MSDocument9 pagesEnergetics Exam Q Booklet MSEmoryNo ratings yet
- Covalent Bonding MSDocument15 pagesCovalent Bonding MSjax stykerNo ratings yet
- Improvement Reason: Above Is Not Essential Any Correct Cycle in Any Possible Answers 0. 11, 0.110, 0.1111 (CL In) OCIDocument63 pagesImprovement Reason: Above Is Not Essential Any Correct Cycle in Any Possible Answers 0. 11, 0.110, 0.1111 (CL In) OCIG M Ali KawsarNo ratings yet
- Number Answer Notes MarksDocument9 pagesNumber Answer Notes MarksAli FahmiNo ratings yet
- Balanced Equations & Associated Calc's 05 MSDocument7 pagesBalanced Equations & Associated Calc's 05 MSlmao lmaoNo ratings yet
- Physical Chemistry 1 MSDocument4 pagesPhysical Chemistry 1 MStomiogunnorinNo ratings yet
- Kinetics BookletDocument5 pagesKinetics BookletSrikaushik TumulaNo ratings yet
- KiougfhiufghhyDocument65 pagesKiougfhiufghhyG M Ali KawsarNo ratings yet
- Above Is Not Essential Any Correct Cycle in Any Possible Answers 0. 11, 0.110, 0.1111 (CL In) OCIDocument63 pagesAbove Is Not Essential Any Correct Cycle in Any Possible Answers 0. 11, 0.110, 0.1111 (CL In) OCIG M Ali KawsarNo ratings yet
- Balanced Equations & Associated Calc's 04 MSDocument9 pagesBalanced Equations & Associated Calc's 04 MSscsabbay16No ratings yet
- Group 1 (Alkali Metals) - Lithium, Sodium, Potassium MSDocument7 pagesGroup 1 (Alkali Metals) - Lithium, Sodium, Potassium MSSanbir SaadNo ratings yet
- KSW FafslfjlsDocument63 pagesKSW FafslfjlsG M Ali KawsarNo ratings yet
- Groups 1 & 2 1 MSDocument11 pagesGroups 1 & 2 1 MSRobbyNo ratings yet
- Cinetica Ejercicios 1Document8 pagesCinetica Ejercicios 1Gustavo OrtizNo ratings yet
- Kinetics MSDocument11 pagesKinetics MSdovidNo ratings yet
- 3 2 Creep PDFDocument7 pages3 2 Creep PDFAZMI 333625No ratings yet
- Bullu AfjlsfjslfjslDocument63 pagesBullu AfjlsfjslfjslG M Ali KawsarNo ratings yet
- Chemsheets GCSE 1279 Calculations Mixture 3 ANSDocument2 pagesChemsheets GCSE 1279 Calculations Mixture 3 ANSJimbo JimboNo ratings yet
- AQA A Level Chem Section 3Document2 pagesAQA A Level Chem Section 3Mahebul MazidNo ratings yet
- Atomic Structure 3 MSDocument10 pagesAtomic Structure 3 MSAHMED MOHAMED MAGDY YAHIA ASHMAWYNo ratings yet
- Chapter 4: Imperfections: Solidification of Molten Metals and AlloysDocument55 pagesChapter 4: Imperfections: Solidification of Molten Metals and AlloysAnnus Abdul GhaffarNo ratings yet
- 6.2ionic Bonding MS - 2Document10 pages6.2ionic Bonding MS - 2Mahir ShahriyarNo ratings yet
- Practice Past Papers DP HL-SL, S 1.1-1.5, S 2.1-2.2, R 2.1 AKDocument15 pagesPractice Past Papers DP HL-SL, S 1.1-1.5, S 2.1-2.2, R 2.1 AKalwafa.q6rNo ratings yet
- Atomic Structure 1 MSDocument9 pagesAtomic Structure 1 MSSheila Ester NyangeNo ratings yet
- 4ch1 1cr Rms 20220825Document16 pages4ch1 1cr Rms 20220825XIN PEINo ratings yet
- 4CH1 2C Rms 20190822Document21 pages4CH1 2C Rms 20190822Åzmâñ Khäñ67% (3)
- 3.6 Periodicity Period 3 Elements and Their Oxides MSDocument15 pages3.6 Periodicity Period 3 Elements and Their Oxides MSmerchantsafa07No ratings yet
- Question Paper - CT-12-PCM-11th-JEE - (Batch-1) - 21.11.2021.pmdDocument12 pagesQuestion Paper - CT-12-PCM-11th-JEE - (Batch-1) - 21.11.2021.pmdBalaji Classes washimNo ratings yet
- A-Level Paper 3 pp8 MsDocument8 pagesA-Level Paper 3 pp8 Ms22S48 SUNDARAM RAMASUBBU RAKSHANo ratings yet
- 7.2covalent Bonding MS - 2Document17 pages7.2covalent Bonding MS - 2Mahir ShahriyarNo ratings yet
- 4CH0 1C MSC 20190307Document23 pages4CH0 1C MSC 20190307WafaNo ratings yet
- Mark Scheme (Results) : Pearson Edexcel International GCSE in Chemistry (4CH0) Paper 2CDocument22 pagesMark Scheme (Results) : Pearson Edexcel International GCSE in Chemistry (4CH0) Paper 2CBlue CanaryNo ratings yet
- Mathematical Negative)Document66 pagesMathematical Negative)G M Ali KawsarNo ratings yet
- Atomic Structure 1 MSDocument8 pagesAtomic Structure 1 MSMohamed EzzatNo ratings yet
- Physics Paper 2 - Marking SchemeDocument12 pagesPhysics Paper 2 - Marking SchemekiruhuravaleryNo ratings yet
- AS Kinetics MSDocument21 pagesAS Kinetics MSvintu pvNo ratings yet
- Part 1 Chapter 3 - Mass Relationships in Chemical ReactionsDocument44 pagesPart 1 Chapter 3 - Mass Relationships in Chemical ReactionsaltheabarlisNo ratings yet
- 1 2 Revision Guide Calculations Aqa PDFDocument14 pages1 2 Revision Guide Calculations Aqa PDFAlisha ShahidNo ratings yet
- HitosisDocument6 pagesHitosisAnela XVIINo ratings yet
- 20.3 F Ma QuestionsDocument1 page20.3 F Ma QuestionsAnela XVIINo ratings yet
- 3.5 Consolidation - Week 03Document9 pages3.5 Consolidation - Week 03Anela XVIINo ratings yet
- Y12 April Assessment 2019Document10 pagesY12 April Assessment 2019Anela XVIINo ratings yet
- Y12 Group 2, 7 and Redox TestDocument10 pagesY12 Group 2, 7 and Redox TestAnela XVIINo ratings yet
- General Chemistry 2 - Q3 - SLM18Document14 pagesGeneral Chemistry 2 - Q3 - SLM18basisterjohnlorenzNo ratings yet
- Sample Chem IaDocument12 pagesSample Chem IaMelis AlparslanNo ratings yet
- Well Intervention - English Metric 10.2 Formula SheetDocument2 pagesWell Intervention - English Metric 10.2 Formula Sheetamri hutabarat100% (1)
- Fundamentals of Physical GeographyDocument29 pagesFundamentals of Physical GeographyHarbinder Singh100% (1)
- 1965 (Q1) - Lessons From Crane RunwaysDocument5 pages1965 (Q1) - Lessons From Crane RunwaysCesar RjszvlNo ratings yet
- KS2-English-Term 2 Revision PaperDocument4 pagesKS2-English-Term 2 Revision PaperCosmos WithmeNo ratings yet
- Series and Parallel PumpDocument15 pagesSeries and Parallel PumpbandarNo ratings yet
- Polymetron 8810-ORP Ion Selective Electrode ISE Calcium Analyzer Installation Procedure PDFDocument13 pagesPolymetron 8810-ORP Ion Selective Electrode ISE Calcium Analyzer Installation Procedure PDFqasim_maqboolNo ratings yet
- FundamentalChromatographicParameters PDFDocument6 pagesFundamentalChromatographicParameters PDFOis GotchaNo ratings yet
- L2S1 Polym. Science Lecture PresentationDocument70 pagesL2S1 Polym. Science Lecture PresentationAruna KumarasiriNo ratings yet
- Sudan III.1390Document2 pagesSudan III.1390Joko JokoNo ratings yet
- Dromus OilDocument1 pageDromus OilSidharth SreedharNo ratings yet
- A Brief Introduction To Composite MaterialsDocument67 pagesA Brief Introduction To Composite Materialsmaddy_scribdNo ratings yet
- High Temperature Corrosion On TurbochargersDocument22 pagesHigh Temperature Corrosion On Turbochargers123turbo100% (2)
- Iit-Jam Geology Free Solved PaperDocument23 pagesIit-Jam Geology Free Solved Paperpankaj_mbmNo ratings yet
- APEX-3D Expert System For Drug DesignDocument7 pagesAPEX-3D Expert System For Drug DesignKhurram RajputNo ratings yet
- LN Series PDFDocument18 pagesLN Series PDFLa Factoría de la CreatividadNo ratings yet
- Poly Aluminium ChlorideDocument2 pagesPoly Aluminium ChlorideanggunNo ratings yet
- Manual mp60 PDFDocument11 pagesManual mp60 PDFAnonymous ie3Xue46No ratings yet
- PVC MsdsDocument3 pagesPVC MsdsSyed OsmanNo ratings yet
- Newsletter May2013Document8 pagesNewsletter May2013GmanNo ratings yet
- Uts Termo Reguler 2014Document2 pagesUts Termo Reguler 2014raharjo1608No ratings yet
- Physical Quantities and UnitsDocument41 pagesPhysical Quantities and UnitsRanjith RajNo ratings yet
- SAMPLE Contents Compilation of Lab ReportsDocument4 pagesSAMPLE Contents Compilation of Lab ReportsJuvyneil CartelNo ratings yet
- CLS JEEAD-19-20 XII Che Target-3 Level-1 Chapter-9Document12 pagesCLS JEEAD-19-20 XII Che Target-3 Level-1 Chapter-9SUNANDAN GUPTANo ratings yet
- CP 04 - Factors Affecting Enzyme Reactions PDFDocument5 pagesCP 04 - Factors Affecting Enzyme Reactions PDFAngry BirdNo ratings yet
- Parameter Proses Produksi ViscoseDocument5 pagesParameter Proses Produksi ViscosePra93No ratings yet