Professional Documents
Culture Documents
1968 - Bennich Et Al. - Immunoglobulin E A New Class of Human Immunoglobulin
Uploaded by
pond_19930 ratings0% found this document useful (0 votes)
4 views2 pagesOriginal Title
1968_Bennich et al._Immunoglobulin E a new class of human immunoglobulin
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views2 pages1968 - Bennich Et Al. - Immunoglobulin E A New Class of Human Immunoglobulin
Uploaded by
pond_1993Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Immunologv, 1968, 15, 323.
Immunoglobulin E: ANew Class of Human Immunoglobulin*
Studies of the nature of the antibodies associated with isologous skin sensitizing activity
have indicated the presence of a previously unrecognized immunoglobulin in human
serum. The immunoglobulin was identified by virtue of specific antigenic determinants.
Independently, a protein with similar antigen characteristics was identified both in the
serum of a patient with multiple myeloma and in normal serum. It is proposed that the
normal protein and antigenically related myeloma proteins shall be designated IgE or
yE, and the heavy polypeptide chains of these molecules be designated E (epsilon)-chains,
in accordance with Nomenclature for Human Immunoglobulins.t This replaces the previous
usage of yE-globulin and IgND.
IgE has antigenic determinants in common with other immunoglobulin classes, as well
as specific antigenic determinants. IgE from non-myeloma sources contained the deter-
minants of light chains of Type K and Type L. The E myeloma protein had light chains
of Type L. The specific antigenic determinants of IgE were not detectable in IgG, IgA,
IgM and IgD. Antisera specific for IgE failed to react with immunoglobulins of these four
classes and their presently recognized subclasses. Conversely antisera specific for these
classes and their subclasses failed to react with IgE.
Studies on the E myeloma protein showed that it contained heavy and light polypeptide
chains with molecular weights of approximately 75,500 and 22,500. The unique deter-
minants of IgE were not present on its light chains. The IgE determinants have not yet
been directly demonstrated on the isolated heavy chains, but are thought to be located
there for the following reasons:
(a) Digestion of the E myeloma protein with papain produced two kinds of fragments,
referred to as Fab- and Fc-fragments by analogy with the fragments produced by the
action of papain on IgG. The Fab-fragment contained light chain determinants but no
IgE determinants. The Fc-fragment lacked light chain determinants. The IgE specific
determinants were found only on this fragment.
(b) Molecular weight data were consistent with the Fc-fragment being a portion of
the heavy chains. The molecular weight of the intact molecule was found to be approxi-
mately 200,000. Following complete reduction and dissociation, 20 per cent of the protein
was recovered as light chains of molecular weight approximately 22,500, indicating
two light chains per molecule. Assuming two heavy chains per molecule the molecular
weight of each heavy chain was calculated to be approximately 75,500. The Fc-fragment
had an approximate molecular weight of 100,000 estimated from sedimentation and gel
filtration data.
Consideration of the antigenic analysis and the physico-chemical data indicated that
IgE determinants were located in the heavy chains of the molecule.
There is evidence for antibody activity in IgE. IgE from selected sera was shown to
combine with a number of antigens using several techniques of radio-immunoassay. The
specificity of these reactions was comparable to the specificity of antigen binding by
other classes of immunoglobulins.
In studies of immediate-type hypersensitivity the binding activity of IgE for a given
* This memorandum was drafted by the signatories (see page 324) following discussions held at the WHO Inter-
national Reference Centre for Immunoglobulins in Lausanne in February 1968.
t Bull. WId Hlth Org., 1964, 30, 447-450.
323
324 Immunoglobulin E
allergen in various human sera correlated with the ability of these sera to passively
sensitize human skin to that allergen. IgE non-reactive with specific allergen and the E
myeloma protein blocked this sensitization. The ability to induce or block isologous
passive skin sensitization may be a characteristic of the human IgE class.
H. H. BENNICH: The Institute of Biochemistry, University of Uppsala, Uppsala, Sweden.
K. ISHIZAKA: Children's Asthma Research Institute and Hospital, Denver, Colorado,
U.S.A.
S. G. O. JOHANSSON: The Blood Centre, University Hospital, Uppsala, Sweden.
D. S. ROWE: Director, WHO International Reference Centre for Immunoglobulins,
Institut de Biochimie, Lausanne, Switzerland.
D. R. STANWORTH: Department of Experimental Pathology, University of Birmingham,
Birmingham, England.
W. D. TERRY: National Cancer Institute, National Institutes of Health, Bethesda,
Maryland, U.S.A.
You might also like
- IGCSE Chemistry Note (9-1) On States of MatterDocument4 pagesIGCSE Chemistry Note (9-1) On States of MatterMd. Saif Ullah Bari100% (3)
- Antigen and AntibodyDocument40 pagesAntigen and Antibodydrdhirenvet100% (1)
- Antibodies: Immunoglobulins - A Family of ProteinsDocument21 pagesAntibodies: Immunoglobulins - A Family of ProteinsMike zombieNo ratings yet
- Route ListDocument168 pagesRoute ListMd Hasnat RabbaniNo ratings yet
- Photochemistry-Ppt 7422144 PowerpointDocument10 pagesPhotochemistry-Ppt 7422144 PowerpointArangaNo ratings yet
- Immunoglobulins - Structure and Function Definition: Immunoglobulins (Ig)Document9 pagesImmunoglobulins - Structure and Function Definition: Immunoglobulins (Ig)Valdez Francis ZaccheauNo ratings yet
- Universal Charging and Testing Unit FPU-1: For Bladder, Piston and Diaphragm AccumulatorsDocument15 pagesUniversal Charging and Testing Unit FPU-1: For Bladder, Piston and Diaphragm AccumulatorsRuben Dario Ortiz FNo ratings yet
- IMS - AntibodiesDocument3 pagesIMS - AntibodiesJeanne Rodiño100% (1)
- ICC IHCbasic20070806Document36 pagesICC IHCbasic20070806api-3821304100% (1)
- Astm C-452Document3 pagesAstm C-452Rafael BorchardtNo ratings yet
- The Human IgG Subclasses: Molecular Analysis of Structure, Function and RegulationFrom EverandThe Human IgG Subclasses: Molecular Analysis of Structure, Function and RegulationFarouk ShakibNo ratings yet
- CHEMICAL LISTINGDocument21 pagesCHEMICAL LISTINGDyeing Dyeing100% (1)
- Stoichiometry Worksheet SolutionsDocument2 pagesStoichiometry Worksheet SolutionsQwert LimNo ratings yet
- (Edexcel International GCSE) Cliff Curtis-Edexcel Igcse Chemistry. Revision Guide. Solution Manual-Pearson Education (2011) PDFDocument16 pages(Edexcel International GCSE) Cliff Curtis-Edexcel Igcse Chemistry. Revision Guide. Solution Manual-Pearson Education (2011) PDFMohamed AlserNo ratings yet
- Study immune system reactions and componentsDocument5 pagesStudy immune system reactions and componentsbayu_wepe82No ratings yet
- NPTEL – Cellular and Molecular Immunology: Antibodies and AntigensDocument33 pagesNPTEL – Cellular and Molecular Immunology: Antibodies and AntigensAygul RamankulovaNo ratings yet
- Antibody Structure and FunctionDocument21 pagesAntibody Structure and Functionemmanuel-u-obi-2256No ratings yet
- The Gell and Coombs classification of hypersensitivity reactionsDocument25 pagesThe Gell and Coombs classification of hypersensitivity reactionsanandiprNo ratings yet
- Abo DescriptionDocument17 pagesAbo DescriptionMohamad IqbalNo ratings yet
- Geb 403chapter 3 (L1) - ImmunoglobulinDocument22 pagesGeb 403chapter 3 (L1) - ImmunoglobulinC JNo ratings yet
- Immunology, ELISADocument9 pagesImmunology, ELISAHằngHamHốNo ratings yet
- Immunoglobulins - AntibodiesDocument10 pagesImmunoglobulins - AntibodiesJesuhovie Solomon OkpobrisiNo ratings yet
- Immunoglobulins - Structure and FunctionDocument8 pagesImmunoglobulins - Structure and FunctionNeha KumarNo ratings yet
- Y20150066 Structure of Immunoglobulins Deeksha Patel-1Document18 pagesY20150066 Structure of Immunoglobulins Deeksha Patel-1deekshapatel.7665No ratings yet
- AntigenDocument75 pagesAntigenAnshuman KatakyNo ratings yet
- Immunology: Dr. A.K.M. Akbar KabirDocument23 pagesImmunology: Dr. A.K.M. Akbar KabirRakib's exploration worldNo ratings yet
- Immunoglobulins/Antibody: Presented By: Shashi Regd. No. 11006142 Roll No. B15 Section RP8003Document17 pagesImmunoglobulins/Antibody: Presented By: Shashi Regd. No. 11006142 Roll No. B15 Section RP8003Shashi SharmaNo ratings yet
- Antibody-Structure, Classes and FunctionsDocument5 pagesAntibody-Structure, Classes and FunctionsNadia UmmahNo ratings yet
- Immunoglobulin Structure and ClassesDocument12 pagesImmunoglobulin Structure and Classes19ph012 Jaya sangeethaNo ratings yet
- Detection of Allergen Specific IgE Antibody ResponsesDocument12 pagesDetection of Allergen Specific IgE Antibody ResponsesYulius DonyNo ratings yet
- Interferons ImmunoglobilinsDocument20 pagesInterferons Immunoglobilinswritters2023No ratings yet
- Antibodies - Immuno AssignmentDocument30 pagesAntibodies - Immuno AssignmentShubham PandeyNo ratings yet
- Structure and Function of Different Class of Immunoglobulins Antigen Antibody InteractionDocument36 pagesStructure and Function of Different Class of Immunoglobulins Antigen Antibody InteractionNikita Singh RaghavNo ratings yet
- Text Size and Immunoglobulin StructureDocument10 pagesText Size and Immunoglobulin StructureViral PatelNo ratings yet
- 1986 - Grassi, Didierlaurent, Stadler - Quantitative Determination of Total and Specific Human IgE With The Use of Monoclonal AntibodiesDocument15 pages1986 - Grassi, Didierlaurent, Stadler - Quantitative Determination of Total and Specific Human IgE With The Use of Monoclonal Antibodiespond_1993No ratings yet
- 6d. Enzyme-Linked ImmunoSorbant Assay (ELISA) - PowerPointDocument31 pages6d. Enzyme-Linked ImmunoSorbant Assay (ELISA) - PowerPointshivani dasNo ratings yet
- Antibody Iggandiga: Presented by Syed - Khaja.Ali Uddin M.S.D (Endodontics)Document39 pagesAntibody Iggandiga: Presented by Syed - Khaja.Ali Uddin M.S.D (Endodontics)Ali SyedNo ratings yet
- Immunoglobulin Structure and FunctionsDocument26 pagesImmunoglobulin Structure and Functionssazia gaurNo ratings yet
- Antigen and Antibodies-2021Document37 pagesAntigen and Antibodies-2021مروه عماد عيسىNo ratings yet
- AntibodyDocument21 pagesAntibodyhogirimmunologyNo ratings yet
- IgG and IgM Antibodies at Lead City UniversityDocument14 pagesIgG and IgM Antibodies at Lead City UniversityVivianNo ratings yet
- Ganong All MCQDocument55 pagesGanong All MCQSagor Kumar DasNo ratings yet
- Popular Chemistryprize2004Document7 pagesPopular Chemistryprize2004manfredm6435No ratings yet
- ImmunoglobulinsDocument9 pagesImmunoglobulinsChathumini YasaraNo ratings yet
- Activity 12 ImmunochemistryDocument7 pagesActivity 12 ImmunochemistryGabrielle John HernaezNo ratings yet
- Antibody Labeling Fluorescence Micros - Fluorescent Immunocytochemistry Fluorescent Immunohistochem - Indirect Immunocytochemistry ImmunostainingDocument6 pagesAntibody Labeling Fluorescence Micros - Fluorescent Immunocytochemistry Fluorescent Immunohistochem - Indirect Immunocytochemistry ImmunostainingDr. Salvador Muñoz BarriosNo ratings yet
- P-3116 - Immunocytochemistry Lecture Notes PDFDocument26 pagesP-3116 - Immunocytochemistry Lecture Notes PDFSwetha RameshNo ratings yet
- Iso Electric Focussing Apparatus-2Document24 pagesIso Electric Focussing Apparatus-2Muhammad AwaisNo ratings yet
- Immunochemistry (2) KordofanDocument45 pagesImmunochemistry (2) KordofanDr. Atif Hassan KhirelsiedNo ratings yet
- Chapter 11 AntibodyDocument86 pagesChapter 11 AntibodyPinaki ChatterjeeNo ratings yet
- MCB 221 - Gen Micro NotesDocument6 pagesMCB 221 - Gen Micro NotesAdams DeborahNo ratings yet
- Antigen Dalam Tiroid AutoimunDocument29 pagesAntigen Dalam Tiroid AutoimunvinoadhiyogaNo ratings yet
- Antibodies Structure and FunctionDocument16 pagesAntibodies Structure and Functionياسين احمد علي الشيخNo ratings yet
- Antibodies: Shashi AnandDocument40 pagesAntibodies: Shashi AnandDil NavabNo ratings yet
- Structure and Functions of Antibody ClassesDocument15 pagesStructure and Functions of Antibody ClassesFaiza RashidNo ratings yet
- immuno3Document5 pagesimmuno3diwanakram83No ratings yet
- Lecture 4: Specific Immunology: Antigen Binding Capacity of The Antibody Will DifferDocument5 pagesLecture 4: Specific Immunology: Antigen Binding Capacity of The Antibody Will DifferMohit ModyNo ratings yet
- J Exp Med-1986-Walsh-41-53Document13 pagesJ Exp Med-1986-Walsh-41-53Seosamh BranaghNo ratings yet
- Immunity: It Is Mediated by Either Antibodies (Humeral Immunity) or Lymphoid Cells (Cellular Immunity) - It Can BeDocument39 pagesImmunity: It Is Mediated by Either Antibodies (Humeral Immunity) or Lymphoid Cells (Cellular Immunity) - It Can BeAmod PrasadNo ratings yet
- Artículo 1 Protein ADocument14 pagesArtículo 1 Protein AdyannissNo ratings yet
- Plasma Proteins and Immunoglobulins GuideDocument41 pagesPlasma Proteins and Immunoglobulins GuideTwinkle SalongaNo ratings yet
- Human Neurocysticercosis: Ige in Cerebrospinal FluidDocument4 pagesHuman Neurocysticercosis: Ige in Cerebrospinal FluidCurandeiro L.No ratings yet
- Heterogeneous T Cell Responses To Beta-Lactam-Modified Self-Structures Are Observed in Penicillin-Allergic IndividualsDocument10 pagesHeterogeneous T Cell Responses To Beta-Lactam-Modified Self-Structures Are Observed in Penicillin-Allergic IndividualsEmail KosongNo ratings yet
- Antibody Production and Purification Technical Handbook, Version 2Document41 pagesAntibody Production and Purification Technical Handbook, Version 2bh14561No ratings yet
- ElectroforesisDocument3 pagesElectroforesisJhonatan Efraín López CarbajalNo ratings yet
- 1990 - Perez Et Al. - cDNA Cloning and Immunological Characterization of The Rye Grass Allergen Lol P IDocument7 pages1990 - Perez Et Al. - cDNA Cloning and Immunological Characterization of The Rye Grass Allergen Lol P Ipond_1993No ratings yet
- 1990 - O'Kennedy Et Al. - Experimental Section A Review of Enzyme-Immunoassay and A Description of A Competitive Enzyme-Linked ImmunosorbentDocument5 pages1990 - O'Kennedy Et Al. - Experimental Section A Review of Enzyme-Immunoassay and A Description of A Competitive Enzyme-Linked Immunosorbentpond_1993No ratings yet
- Pi I 0091674987902867Document11 pagesPi I 0091674987902867Elaine MedeirosNo ratings yet
- 1989 - Gierasch - Signal SequencesDocument8 pages1989 - Gierasch - Signal Sequencespond_1993No ratings yet
- 1989 - Cookson Et Al. - Linkage Between Immunoglobulin E Responses Underlying Asthma and Rhinitis and Chromosome 11qDocument4 pages1989 - Cookson Et Al. - Linkage Between Immunoglobulin E Responses Underlying Asthma and Rhinitis and Chromosome 11qpond_1993No ratings yet
- 1991 - Badya, Pasha - A Pollen Calendar For Chittagong University Campus, Chittagong (Bangladesh)Document7 pages1991 - Badya, Pasha - A Pollen Calendar For Chittagong University Campus, Chittagong (Bangladesh)pond_1993No ratings yet
- 1992 - McQueen-Mason, Durachko, Cosgrove - Two Endogenous Proteins That Induce Cell Wall Extension in PlantsDocument10 pages1992 - McQueen-Mason, Durachko, Cosgrove - Two Endogenous Proteins That Induce Cell Wall Extension in Plantspond_1993No ratings yet
- 1991 - Chang Et Al. - Analysis of Allergenic Components of Bermuda Grass Pollen by Monoclonal AntibodiesDocument10 pages1991 - Chang Et Al. - Analysis of Allergenic Components of Bermuda Grass Pollen by Monoclonal Antibodiespond_1993No ratings yet
- 1983 - Ruffin Et Al. - The Effects of Some Environmental Gaseous Pollutants On Pollen Wall Proteins of Certain Airborne Pollen Grains A PRDocument6 pages1983 - Ruffin Et Al. - The Effects of Some Environmental Gaseous Pollutants On Pollen Wall Proteins of Certain Airborne Pollen Grains A PRpond_1993No ratings yet
- 1982 - Platts-Mills Et Al. - Reduction of Bronchial Hyperreactivity During Prolonged Allergen AvoidanceDocument4 pages1982 - Platts-Mills Et Al. - Reduction of Bronchial Hyperreactivity During Prolonged Allergen Avoidancepond_1993No ratings yet
- 1986 - Plebani Et Al. - An Enzyme-Linked Immunosorbent Assay For Cow's Milk Protein-Specific IgE Using Biotinylated AntigenDocument6 pages1986 - Plebani Et Al. - An Enzyme-Linked Immunosorbent Assay For Cow's Milk Protein-Specific IgE Using Biotinylated Antigenpond_1993No ratings yet
- Sporopollenin A Review of Its Chemistry Palaeochemistry and GeochemistryDocument8 pagesSporopollenin A Review of Its Chemistry Palaeochemistry and GeochemistryAnargha BoseNo ratings yet
- 1984 - Von Heijne - How Signal Sequences Maintain Cleavage SpecificityDocument9 pages1984 - Von Heijne - How Signal Sequences Maintain Cleavage Specificitypond_1993No ratings yet
- 1981 - Merrett - The Radioallergosorbent Test (RAST)Document6 pages1981 - Merrett - The Radioallergosorbent Test (RAST)pond_1993No ratings yet
- 1981 - Norman, Lichtenstein, Marsh - Studies On Allergoids From Naturally Occurring Allergens IV. Efficacy and Safety of Long-Term AllergoidDocument11 pages1981 - Norman, Lichtenstein, Marsh - Studies On Allergoids From Naturally Occurring Allergens IV. Efficacy and Safety of Long-Term Allergoidpond_1993No ratings yet
- 1982 - Bayne, Mathews - Determination of Total IgE by ELISA in Tubes and Plates Compared With PRISTDocument3 pages1982 - Bayne, Mathews - Determination of Total IgE by ELISA in Tubes and Plates Compared With PRISTpond_1993No ratings yet
- 1953 - Samter, Kofoed, Pieper - A Factor in Lungs of Anaphylactically Shocked Guinea Pigs Which Can Induce Eosinophilia in Normal AnimalsDocument13 pages1953 - Samter, Kofoed, Pieper - A Factor in Lungs of Anaphylactically Shocked Guinea Pigs Which Can Induce Eosinophilia in Normal Animalspond_1993No ratings yet
- 1961 - Voorhorst - The Eosinophil Cell and Its Role in Allergy and in InfectionDocument20 pages1961 - Voorhorst - The Eosinophil Cell and Its Role in Allergy and in Infectionpond_1993No ratings yet
- Swet 2Document4 pagesSwet 2pond_1993No ratings yet
- 1976 - Ohman Et Al. - Allergens of Mammalian OriginDocument11 pages1976 - Ohman Et Al. - Allergens of Mammalian Originpond_1993No ratings yet
- 1976 - Johansson, Berglund, Kjellman - Comparison of IgE Values As Determined by Different Solid Phase Radioimmunoassay MethodsDocument9 pages1976 - Johansson, Berglund, Kjellman - Comparison of IgE Values As Determined by Different Solid Phase Radioimmunoassay Methodspond_1993No ratings yet
- 1972 - Parish - Eosinophilia. II. Cutaneous Eosinophilia in Guinea-Pigs Mediated by Passive Anaphylaxis With IgGl or Reagin, and Antigen-AntDocument16 pages1972 - Parish - Eosinophilia. II. Cutaneous Eosinophilia in Guinea-Pigs Mediated by Passive Anaphylaxis With IgGl or Reagin, and Antigen-Antpond_1993No ratings yet
- 1951 - Herxheimer - Induced Asthma in ManDocument5 pages1951 - Herxheimer - Induced Asthma in Manpond_1993No ratings yet
- BONDUS - Structure Test 2Document4 pagesBONDUS - Structure Test 2PatriciaRejasCalvoNo ratings yet
- How Does The Body Switch Between Aerobic and Anaerobic Respiration? - Biology Stack ExchangeDocument12 pagesHow Does The Body Switch Between Aerobic and Anaerobic Respiration? - Biology Stack Exchangepond_1993No ratings yet
- The Role of Vitamins and Minerals in Energy MetaboDocument15 pagesThe Role of Vitamins and Minerals in Energy MetaboWida SarjiahNo ratings yet
- Tofel Reading Comprehension Practice QuestionsDocument4 pagesTofel Reading Comprehension Practice Questionspond_1993No ratings yet
- 9510ưưDocument5 pages9510ưưpond_1993No ratings yet
- Cellular Respiration: ATP Generation and StepsDocument2 pagesCellular Respiration: ATP Generation and Stepspond_1993No ratings yet
- Normalab GlassW PetroDocument24 pagesNormalab GlassW Petrokevin100% (1)
- G9 Chem Paper 4Document7 pagesG9 Chem Paper 4harshvaardhanNo ratings yet
- ASTM D1784 - Jtvo9242Document4 pagesASTM D1784 - Jtvo9242Nayth Andres GalazNo ratings yet
- NewTiger4 U6 BasicsWritingPracticeDocument1 pageNewTiger4 U6 BasicsWritingPracticeANDREA ESTEBAN MARTINEZNo ratings yet
- PVC LS100 by LGDocument1 pagePVC LS100 by LGabhaygupta1No ratings yet
- CH 501-Lecture-01Document56 pagesCH 501-Lecture-01Vihanga SenanayakeNo ratings yet
- Effects of High Calcium Concentration On The Development of Methanogenic Sludge in Upflow Anaerobic Sludge Bed ReactorsDocument9 pagesEffects of High Calcium Concentration On The Development of Methanogenic Sludge in Upflow Anaerobic Sludge Bed ReactorsjafarshodiqqNo ratings yet
- Shanghai Test Results - Filtersafe White PaperDocument21 pagesShanghai Test Results - Filtersafe White PaperBlack PantherNo ratings yet
- A - Lab - Chem EquilibDocument4 pagesA - Lab - Chem EquilibshayneNo ratings yet
- Law of Conservation of Mass QuizDocument6 pagesLaw of Conservation of Mass QuizLeormhan Jacob Dela CruzNo ratings yet
- State-of-the-Art Report: Bond Under Cyclic Loads: ACI 408.2R-92Document5 pagesState-of-the-Art Report: Bond Under Cyclic Loads: ACI 408.2R-92satheeshNo ratings yet
- Taxation of Business Entities 2017 8th Edition Spilker Solutions ManualDocument25 pagesTaxation of Business Entities 2017 8th Edition Spilker Solutions ManualLukeCamerondepo100% (34)
- Lesson 8 AlkenesDocument10 pagesLesson 8 AlkenesSideka ResalsinghNo ratings yet
- TDS OPT Accelerator 1-Aug15 PDFDocument1 pageTDS OPT Accelerator 1-Aug15 PDFTamer BidakNo ratings yet
- QA-QC TPL of Ecube LabDocument1 pageQA-QC TPL of Ecube LabManash Protim GogoiNo ratings yet
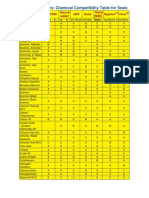
- Chemical Compatibility Table For SealsDocument3 pagesChemical Compatibility Table For SealsThanh Tuyên VõNo ratings yet
- Correcting Configurations: Learning ObjectivesDocument3 pagesCorrecting Configurations: Learning ObjectivesZainab FahadNo ratings yet
- MSDS ChapsDocument1 pageMSDS ChapsPeterNo ratings yet
- Psfsiee ReviewDocument10 pagesPsfsiee Reviewlimichael000No ratings yet
- All-in-One Study Material for Boards & Entrance ExamsDocument46 pagesAll-in-One Study Material for Boards & Entrance Examsara_anjo100% (1)
- _EXP 9 KUT101Document17 pages_EXP 9 KUT101hannan sharizalNo ratings yet
- Micron Filter BagDocument2 pagesMicron Filter Bagfilter fabricNo ratings yet