Professional Documents
Culture Documents
Types of Fuel Cells
Uploaded by
Juan Antonio Sánchez0 ratings0% found this document useful (0 votes)
4 views1 pageCopyright
© © All Rights Reserved
Available Formats
XLS, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as XLS, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views1 pageTypes of Fuel Cells
Uploaded by
Juan Antonio SánchezCopyright:
© All Rights Reserved
Available Formats
Download as XLS, PDF, TXT or read online from Scribd
You are on page 1of 1
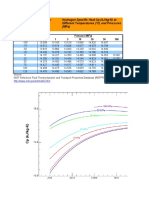
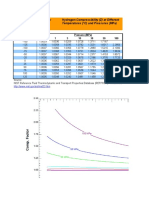
Hydrogen Analysis Resource Center: Types of Fuel Cells - Descriptions and Characteristics
Types of Fuel Reactions Electrolyte Applications Advantages Disadvantages
Cells Material
A: Anode
C: Cathode
PEM (polymer A: H2 → 2H+ + 2e- proton- •portable power •solid construction •sensitive to impurities
electrolyte C: ½ O2 + 2H+ + 2e- → H2O conducting •transportation •operate at relatively low •precious metal catalysts
membrane fuel polymer temperatures (about
cell) 200ºF).
•stationary power •H2 fuel
SOFC (solid oxide A: H2 + O= → H2O + 2e- oxide ion- •stationary power •used with many fuels •very high temperature
fuel cell) conducting (1800ºF)
CO + O= → CO2 + 2e- ceramic •semi trucks •solid and rugged •expensive materials
CH4 + 4O= → 2H2O + CO2 + 8e-
C: ½ O2 + 2e- → O=
MCFC (molten A: H2 + CO3= → H2O + CO2 + 2e- molten •power plants •used with many fuels •very corrosive electrolyte
carbonate fuel cell) carbonate salt
CO + CO3= → 2CO2 + 2e- in a ceramic •high efficiency •high temperature (about
1200ºF)
C: ½ O2 + CO2 + 2e- → CO3= •precious metal catalysts
AFC (alkaline fuel A:H2 + 2(OH)- → 2H2O + 2e- aqueous •power and water •efficiencies of up to 70% •sensitive to carbon dioxide
cell) C: ½ O2 + H2O + 2e- → 2(OH)- potassium •space vehicles •very caustic medium
hydroxide
•H2 fuel
PAFC (phosphoric A: H2 → 2H+ + 2e- phosphoric •stationary power •most commercially •very corrosive electrolyte
acid fuel cell) acid in a developed fuel cell
C:½ O2 + 2H+ + 2e- → H2O matrix •power plants •operating temperature •unstable at higher
range of PAFC is 400ºF. temperatures
•H2 fuel
Source:
EG&G Technical Services, Inc, Fuel Cells Handbook; 7th Edition, November 2004 available at: http://www.seca.doe.gov/pubs/FCHandbook7.pdf
You might also like
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Thesis EnergianDocument237 pagesThesis EnergianJuan Antonio SánchezNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5807)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Hydrogen PropertiesDocument1 pageHydrogen PropertiesJuan Antonio SánchezNo ratings yet
- Cs Distr Steam Methane ReformDocument5 pagesCs Distr Steam Methane ReformJuan Antonio SánchezNo ratings yet
- Total Hydrogen Production Us EuropeDocument1 pageTotal Hydrogen Production Us EuropeJuan Antonio SánchezNo ratings yet
- SI PrefixesDocument2 pagesSI PrefixesJuan Antonio SánchezNo ratings yet
- Physical ConstantsDocument1 pagePhysical ConstantsJuan Antonio SánchezNo ratings yet
- Specific Heat (SI)Document6 pagesSpecific Heat (SI)Juan Antonio SánchezNo ratings yet
- Energy Equivalence of FuelsDocument1 pageEnergy Equivalence of FuelsJuan Antonio SánchezNo ratings yet
- Compressibility (SI)Document6 pagesCompressibility (SI)Juan Antonio SánchezNo ratings yet
- Thermal Conductivity (SI)Document6 pagesThermal Conductivity (SI)Juan Antonio SánchezNo ratings yet
- Properties of Hydrogen NBPDocument2 pagesProperties of Hydrogen NBPJuan Antonio SánchezNo ratings yet
- Enthalpy (SI)Document6 pagesEnthalpy (SI)Juan Antonio SánchezNo ratings yet
- Lower and Higher Heating ValuesDocument1 pageLower and Higher Heating ValuesJuan Antonio SánchezNo ratings yet
- Entropy (SI)Document6 pagesEntropy (SI)Juan Antonio SánchezNo ratings yet
- HS - Chap6 - Legal Requirements and Standards - V1p2Document27 pagesHS - Chap6 - Legal Requirements and Standards - V1p2Juan Antonio SánchezNo ratings yet
- Comparative - Properties of H2 and FuelsDocument2 pagesComparative - Properties of H2 and FuelsJuan Antonio SánchezNo ratings yet
- HS - Chap7 - Conclusions - Version 1 - 0 - 0Document4 pagesHS - Chap7 - Conclusions - Version 1 - 0 - 0Juan Antonio SánchezNo ratings yet
- HS - Chap1 - H2 Fundamentals - V1p2Document12 pagesHS - Chap1 - H2 Fundamentals - V1p2Juan Antonio SánchezNo ratings yet
- HS - Chap4 - Risk Assesment - V1p2Document24 pagesHS - Chap4 - Risk Assesment - V1p2Juan Antonio SánchezNo ratings yet