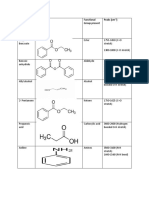
Professional Documents
Culture Documents
Appendix 1 - APR 2011
Uploaded by
Siti Hawa Zakaria0 ratings0% found this document useful (0 votes)
5 views1 pageFTIR Appendix of chemical structure
Original Title
Appendix 1- APR 2011
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentFTIR Appendix of chemical structure
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views1 pageAppendix 1 - APR 2011
Uploaded by
Siti Hawa ZakariaFTIR Appendix of chemical structure
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 1
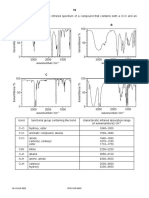
CONFIDENTIAL APPENDIX 1 AS/JAN2012/CHM571
M
SOME CHARACTERISTICS OF IR ABSORPTION BANDS
Wavenumber, cm-1 Intensity Structure
730 - 770; 690 - 710 m Mono (aromatic)
1090 - 1400 s C O (in ethers, alcohols and esters)
1315 - 1475 m-s C H (in alkanes)
C = C bond in aromatic ring (usually shows
1450 - 1600 s
several peaks)
1620 - 1680 m C=C
2100 - 2200 m CC
1690 - 1750 s C = O (in carbonyl compounds and esters)
1740 - 1725 s C = O (in aldehydes)
1700 - 1730 s C = O (in carboxylic acids)
1320 - 1210 m C O (in carboxylic acids)
2760 - 2700
w C H (of aldehyde group, a pair of bands)
2860 - 2800
2400 - 3400 s, vb O H in COOH
3000 - 3100 m C H (C is part of aromatic ring)
3300 s C H (sp)
3020 - 3080 m C H (sp2)
2800 - 3000 m-s C H (sp3)
3200 - 3600 s, b O H (in H-bonded ROH and ArOH)
Intensities:
s = strong m = medium w = weak b = broad vb = very broad
h = 6.63 x 10-34 J.s
c = 3.00 x 108 ms-1
© Hak Cipta Universiti Teknologi MARA CONFIDENTIAL
You might also like
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Audio IC Circuits Manual: Newnes Circuits Manual SeriesFrom EverandAudio IC Circuits Manual: Newnes Circuits Manual SeriesRating: 5 out of 5 stars5/5 (1)
- Spektro IRDocument64 pagesSpektro IRAnonymous NSK4nvH4ufNo ratings yet
- Infrared Spectra Reveal Molecular StructuresDocument51 pagesInfrared Spectra Reveal Molecular StructuresAakshi JairathNo ratings yet
- Infrared SpectrosDocument51 pagesInfrared SpectrosDrHamdy KhameesNo ratings yet
- Characteristic Infrared Absorption Frequencies Bond Compound Type Frequency Range, CMDocument1 pageCharacteristic Infrared Absorption Frequencies Bond Compound Type Frequency Range, CMTikyo JoNo ratings yet
- Spektrometri IRDocument51 pagesSpektrometri IRClarion 642No ratings yet
- Table - 1: Characteristic Infrared Absorptions of Functional GroupsDocument1 pageTable - 1: Characteristic Infrared Absorptions of Functional GroupsAJIT CHAUDHARINo ratings yet
- Functional Class Range (CM) Intensity Assignment Alkanes: AlkenesDocument1 pageFunctional Class Range (CM) Intensity Assignment Alkanes: AlkenesStoica AlexandruNo ratings yet
- Table 1. Characteristic IR Absorption Peaks of Functional Groups Vibration Position (CM) Intensity Notes Alkanes AlkenesDocument6 pagesTable 1. Characteristic IR Absorption Peaks of Functional Groups Vibration Position (CM) Intensity Notes Alkanes AlkenesBag VatiNo ratings yet
- Spectroscopy RangeDocument4 pagesSpectroscopy Rangematt_drakul4860No ratings yet
- IR Spectra AnalysisDocument37 pagesIR Spectra AnalysisdevoydouglasNo ratings yet
- Table 1. Characteristic IR Absorption Peaks of Functional Groups Vibration Position (CM) Intensity Notes Alkanes AlkenesDocument2 pagesTable 1. Characteristic IR Absorption Peaks of Functional Groups Vibration Position (CM) Intensity Notes Alkanes AlkenesDrBipin DevaniNo ratings yet
- IR ChartDocument2 pagesIR ChartNadiaa SafirraNo ratings yet
- IR Absorption FrequenciesDocument1 pageIR Absorption FrequenciesRismayani Miftahul INo ratings yet
- Infrared (IR) Spectroscopy: Structure, Purity, and IdentityDocument16 pagesInfrared (IR) Spectroscopy: Structure, Purity, and IdentityDiana KowsariNo ratings yet
- Printable Acrobat PDF File: Table of Characteristic IR AbsorptionsDocument3 pagesPrintable Acrobat PDF File: Table of Characteristic IR AbsorptionsImam Hadillah MuhfiNo ratings yet
- Instrumental Methods Appendix - IrDocument8 pagesInstrumental Methods Appendix - IrMạnh BùiNo ratings yet
- 05 IR Chart PDFDocument1 page05 IR Chart PDFKonstantina MsNo ratings yet
- 05 IR Chart PDFDocument1 page05 IR Chart PDFojasvapal singhNo ratings yet
- IR spectroscopy guide for organic functional group analysisDocument24 pagesIR spectroscopy guide for organic functional group analysisakshantratwanNo ratings yet
- 5321 - 1. Materi IrDocument21 pages5321 - 1. Materi IrMega KhoerunissaNo ratings yet
- FTIR TablesDocument1 pageFTIR TablesvandykavidurgaNo ratings yet
- Sample: Structure Functional Group Present Peaks (CM)Document2 pagesSample: Structure Functional Group Present Peaks (CM)MUHAMMAD USMANNo ratings yet
- Sample: Structure Functional Group Present Peaks (CM)Document2 pagesSample: Structure Functional Group Present Peaks (CM)MUHAMMAD USMANNo ratings yet
- IR-freq CO BondDocument3 pagesIR-freq CO BondRD's AcademyNo ratings yet
- Advanced PH Analytics Lec 4Document32 pagesAdvanced PH Analytics Lec 4knowlegebook6No ratings yet
- 2230L 08 IR Spectra InterpretationDocument11 pages2230L 08 IR Spectra Interpretationvennilaj23No ratings yet
- 424 Spectra TablesDocument19 pages424 Spectra TablespradeepiitdNo ratings yet
- Spectroscopic Data Tables for Organic Structure DeterminationDocument4 pagesSpectroscopic Data Tables for Organic Structure Determinationignacio erazoNo ratings yet
- IR Spectroscopy IntroductionDocument14 pagesIR Spectroscopy IntroductionKasani Tirumala tejaNo ratings yet
- Spectratables IR Dan NMRDocument2 pagesSpectratables IR Dan NMRSiska AnggreiniNo ratings yet
- IR and NMR TablesDocument2 pagesIR and NMR TablesMohamed DahmaneNo ratings yet
- Spectroscopy Infrared SpectraDocument51 pagesSpectroscopy Infrared Spectrathanasa08No ratings yet
- 884 - IR Spectroscopy-62-70Document9 pages884 - IR Spectroscopy-62-705amityadavmistoreNo ratings yet
- Infrared Spectroscopy: Conformational IsomersDocument7 pagesInfrared Spectroscopy: Conformational IsomersRiyan NazarudinNo ratings yet
- Ftir Lab ReportDocument7 pagesFtir Lab ReportZharifah Bari'ah Basa'ah100% (1)
- Characteristic IR Absorption FrequenciesDocument2 pagesCharacteristic IR Absorption FrequenciesSumonNo ratings yet
- Adobe Scan 31 May 2023Document1 pageAdobe Scan 31 May 2023Imuu IsmuuNo ratings yet
- Key HW 3 Part II SpecDocument16 pagesKey HW 3 Part II SpecTha KantanaNo ratings yet
- IR and NMR analysis of organic compoundsDocument102 pagesIR and NMR analysis of organic compoundsĐức NamNo ratings yet
- IR Spectroscopy Tables SummaryDocument15 pagesIR Spectroscopy Tables SummaryYuppie RajNo ratings yet
- Esm Infrared SpectrophotometerDocument11 pagesEsm Infrared SpectrophotometerNuralia Radiani RustamNo ratings yet
- Functional Class Range (NM) Intensity Assignment Range (NM) Intensity AssignmentDocument6 pagesFunctional Class Range (NM) Intensity Assignment Range (NM) Intensity AssignmentdubstepoNo ratings yet
- 16 40 Which Diagram Shows The Infrared Spectrum of A Compound That Contains Both A C O and AnDocument1 page16 40 Which Diagram Shows The Infrared Spectrum of A Compound That Contains Both A C O and AnZianNo ratings yet
- Lecture 4 IR Spectrum AnalysisDocument43 pagesLecture 4 IR Spectrum AnalysiskhadijahhannahNo ratings yet
- Frequency Table For IR & NMRDocument6 pagesFrequency Table For IR & NMRYogesh PingleNo ratings yet
- Infrared Spectroscopy Table: Functional Group Frequency (cm-1) IntensityDocument1 pageInfrared Spectroscopy Table: Functional Group Frequency (cm-1) IntensityKezia PatriciaNo ratings yet
- Infrared Spectroscopy Lab GuideDocument5 pagesInfrared Spectroscopy Lab GuideShubham BobadeNo ratings yet
- Infra Red Infra Red: Atmanto Heru WDocument15 pagesInfra Red Infra Red: Atmanto Heru WSetya Dhana Santika AjiNo ratings yet
- IR&NMR ProblemsDocument43 pagesIR&NMR ProblemsAndrew Ronaldi Tandio100% (2)
- Spec Ir NMR Spectra TablesDocument15 pagesSpec Ir NMR Spectra TablesMah NovaesNo ratings yet
- Tabla IRDocument1 pageTabla IR01jchNo ratings yet
- Spectral Interpretation GuideDocument15 pagesSpectral Interpretation Guidemariam nawabNo ratings yet
- Spectroscopy Techniques for Structure AnalysisDocument39 pagesSpectroscopy Techniques for Structure AnalysisJames Baben0% (1)
- IR Lesson 2: The IR Spectra and IR PeaksDocument4 pagesIR Lesson 2: The IR Spectra and IR Peaksanuprathap2006No ratings yet
- BÀI TẬP MẪUDocument13 pagesBÀI TẬP MẪUMinh Tân LêNo ratings yet
- Lecture IR4Document58 pagesLecture IR4Gerges SamirNo ratings yet
- Ion Beams for Materials AnalysisFrom EverandIon Beams for Materials AnalysisR. Curtis BirdNo ratings yet