Professional Documents
Culture Documents
Psychedelic Therapy: A Roadmap For Wider Acceptance and Utilization
Uploaded by
Huy HuoylongOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Psychedelic Therapy: A Roadmap For Wider Acceptance and Utilization
Uploaded by
Huy HuoylongCopyright:
Available Formats
comment
Environments for clinical data, and Molecular Biology Laboratory EMBL-EBI, South 10. Waterhouse, A. et al. Nucleic Acids Res. 46, W296–W303 (2018).
11. UniProt Consortium. Nucleic Acids Res. 49, D480–D489 (2021).
worldwide, the Global Alliance for Global Building, Wellcome Genome Campus, Hinxton, UK. 12. Laskowski, R. A., Jablonska, J., Pravda, L., Varekova, R. S. &
Health is establishing standards and ✉e-mail: thornton@ebi.ac.uk Thornton, J. M. Protein Sci. 27, 129–134 (2018).
protocols to enable swifter progress. For this 13. Mirdita, M., Ovchinnikov, S. & Steinegger, M. Preprint at
https://doi.org/10.1101/2021.08.15.456425 (2021).
to be successful, multi-disciplinary teams Published online: 12 October 2021
14. Baek, M. et al. Science 373, 871–876 (2021).
will be needed, involving clinicians, domain https://doi.org/10.1038/s41591-021-01533-0 15. Batool, M., Ahmad, B. & Choi, S. Int. J. Mol. Sci. 20, 2783 (2019).
experts and machine learning experts, to (11).
16. Stefl, S. et al. J. Mol. Biol. 425, 3919–3936 (2013).
develop the tools to exploit the data. References 17. Landrum, M. J. et al. Nucleic Acids Res. 48, D835–D844 (2020).
It has taken many years to establish the 1. CASP14—14th Community Wide Experiment on the Critical
18. COVID-19 protein structures in the PDB. Retrieved from
Assessment of Techniques for Protein Structure Prediction.
biological databases that are so widely used Retrieved from https://predictioncenter.org/casp14/ (accessed 27
https://www.ebi.ac.uk/thornton-srv/databases/pdbsum/covid-19.
html (accessed 3 September 2021).
today—and the challenge for clinical data September 2021).
19. Lewin, H. A. et al. Proc. Natl Acad. Sci. USA 115, 4325–4333
is even larger. This calls for immediate 2. Jumper, J. et al. Nature 596, 583–589 (2021).
(2018).
3. Tunyasuvunakool, K et al. Nature 595, 590–596 (2021).
investment in creating a new health data 4. Jones, D. T. & Thornton, J. M. Crystallogr. News 156, 6–9 (2021).
20. Darwin Tree of Life. Retrieved from https://www.darwintreeoflife.
org (accessed 27 September 2021).
infrastructure so that patients will be 5. AlQuraishi, M. Curr. Opin. Chem. Biol. 65, 1–8 (2021).
proud to contribute their data to improve 6. Diwan, G. D. et al. J. Mol. Biol. 167180 (2021).
7. Workman, P. The Institute of Cancer Research blogs 2021. Author contributions
human health and the world can face new Retrieved from https://www.icr.ac.uk/blogs/the-drug-discoverer/ J.M.T. wrote the first draft of the article, and R.A.L. and
pandemics with confidence. ❐ page-details/reflecting-on-deepmind-s-alphafold-artificial- N.B. edited and improved it. R.A.L. performed the analyses
intelligence-success-what-s-the-real-significance-for-protein- and created the figures.
Janet M. Thornton ✉, folding-research-and-drug-discovery
8. wwPDB Consortium. Nucleic Acids Res. 47, D520–D528 (2019).
Roman A. Laskowski and Neera Borkakoti 9. Hopkins, A. L. & Groom, C. R. Nat. Rev. Drug. Discov. 1, Competing interests
European Bioinformatics Institute - European 727–730 (2002). J.M.T. sits on the board of Health Data Research UK.
Psychedelic therapy: a roadmap for wider
acceptance and utilization
Psychedelics have shown great promise in treating mental-health conditions, but their use is severely limited by
legal obstacles, which could be overcome.
Mason Marks and I. Glenn Cohen
T
he COVID-19 pandemic has synthesized in the early 20th century. By the from fungi5. Unlike other schedule I
exacerbated a national mental-health middle of the 20th century, clinicians used substances such as heroin, and schedule II
crisis in the United States. For two psychedelics as adjuncts to psychotherapy, compounds, including cocaine and fentanyl,
decades, drug-overdose deaths have risen reporting a variety of benefits. However, in psilocybin exhibits a low risk of toxicity
exponentially, and suicide rates have steadily the 1970s they were categorized as schedule and a very low potential for dependence
increased. These trends reflect deep-seated I controlled substances, which are said to or addiction6. Psilocybin use is not
problems with the healthcare system, have “no currently accepted medical use criminalized in several countries, including
including low investment in preventative and a high potential for abuse”; this blocked Portugal and the Netherlands, and a study
mental healthcare and a lack of innovation mainstream research on these compounds commissioned by the Dutch Ministry of
in psychiatry. In search of more effective for decades. Health found that over-the-counter sales
treatments, clinicians are exploring the In the late 1990s, the US Drug posed minimal risk to individual people and
therapeutic use of psychedelic compounds, Enforcement Administration (DEA) the public7.
a promising avenue for addressing the permitted some researchers to study limited Acknowledging its therapeutic benefits,
mental-health crisis. However, there amounts of psychedelics, which allowed the Canadian government made psilocybin
are social and legal obstacles to making research to resume. Clinical trials have now available to people with life-threatening
psychedelics a viable treatment option1. been conducted at leading universities, and illness through compassionate-use
a growing body of evidence supports the regulation. On the basis of clinical-trial
Schedule I controlled substances use of psychedelics, such as psilocybin and data, the US Food and Drug Administration
Psychedelics are a class of natural MDMA, in the treatment of depression2, (FDA) designated psilocybin a breakthrough
and synthetic compounds that post-traumatic stress disorder3 and anxiety therapy for major depressive disorder and
includes psilocybin, MDMA toward the end of life4. treatment-resistant depression8.
(3,4-methylenedioxymethamphetamine), The schedule I status of most Rescheduling can occur through several
ibogaine and DMT (dimethyltryptamine). psychedelics imposes a ceiling on many means. The US Congress can amend the
Some psychedelics have been used by policy recommendations. The evidence Controlled Substances Act, changing the
Indigenous communities for hundreds in support of rescheduling is strong, categorization of any controlled substance9.
or thousands of years. Others were first particularly for psilocybin, which is derived Alternatively, the president or the federal
Nature Medicine | VOL 27 | October 2021 | 1666–1671 | www.nature.com/naturemedicine 1669
comment
such as the British pharmaceutical firm
Compass Pathways have sought patents
on psilocybin compounds and methods
of treating a variety of mental-health
conditions with psychedelics14. They argue
that patents are necessary to protect their
investments not only in drug discovery
but also in commercialization, which may
involve expensive clinical trials and other
requirements to obtain approval from the
FDA and other regulators and buy-in from
the medical community15.
At the same time, the sudden interest
in patenting psychedelics has prompted
criticism from stakeholders, including
patient advocates, scientists, journalists,
lawyers and Indigenous communities16.
Some claim patenting psychedelics exploits
the traditional knowledge of Indigenous
communities without acknowledgment or
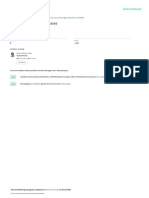
Psilocybin mushrooms. Credit: gre jak / Alamy Stock Photo
compensation, a practice called ‘biopiracy’.
Others argue that patents make
a small number of companies gatekeepers
attorney general could command the Institute on Drug Abuse funded a trial to for the emerging psychedelics industry,
DEA to reschedule a substance. Finally, investigate the use of psilocybin for smoking which could inhibit research, stifle
any person from within or outside the cessation, possibly reflecting an encouraging innovation and restrict access to
government can petition the DEA to policy shift. needed therapies.
reschedule substances, which may trigger Under existing regulation, well-capitalized These concerns are not unique
FDA review of available evidence. private companies fund most research and, to psychedelics. Patents on genetic
The FDA is obligated to protect the to a large extent, they control the agenda and technologies, cancer therapies and other
public and ensure the dissemination of shape federal drug policies. Instead, the goal innovations have engendered similar
accurate medical information, and it should be a psychedelics industry in which debates17. However, some features of
has spontaneously opined on potential patients and marginalized communities have psychedelics, including their long and
scheduling of unregulated substances, such seats at the table. Achieving this goal will complicated history, raise unique concerns
as the dietary supplement kratom. Similarly, require more-inclusive clinical trials and that could exacerbate pre-existing problems
it could recommend the rescheduling of unbiased regulatory review of psychedelics with patenting medical products.
psilocybin because the available evidence no by the FDA. Novelty and non-obviousness are two
longer supports its current classification. The FDA currently oversees phase conditions for patentability. However, because
3 trials of MDMA for the treatment of psychedelics are often derived from natural
Limits on federal funding and research post-traumatic stress disorder and phase products that have been used in traditional
Due to the schedule I status of most 2 trials of psilocybin for the treatment of practices for centuries, psychedelic inventions
psychedelics, federal funding for research is drug-resistant depression11. In addition to may lack novelty or would have been
nearly non-existent. being funded by private donors, existing trials obvious to people experienced in the field.
More directly, a federal appropriations often lack diversity and exclude populations Nevertheless, the US Patent and Trademark
rider — a provision inserted into a funding who may benefit from psychedelics, such as Office (PTO) has issued psychedelic patents
bill that may effectuate public policy by people with histories of severe trauma and of questionable validity18.
limiting how funds are spent — creates self-harm12. An infusion of federal funds Weak psychedelic patents could
a considerable obstacle to such research. could be used to make psychedelics research potentially be invalidated in court, but that
First enacted in 1996, the rider prohibits more equitable and inclusive. does not make them harmless, because patent
federal funds from supporting “any activity holders can still wield them offensively.
that promotes the legalization of any drug Patents may limit access Defending against patent-infringement
or other substance included in schedule Given promising clinical-trial results, claims is expensive, and the prospect
I.”10 This rider has been renewed in every many stakeholders are attempting to patent discourages action by smaller startups and
appropriations process since then. Because psychedelic compounds and methods of non-profit research organizations, even when
research on psychedelics could advance producing and administering them. they are in the right.
scientific knowledge and provide evidence Patents entitle their holders to exclude One explanation for the issuance of
that supports rescheduling, a form of others from making, using or selling problematic patents for psychedelics may
legalization, the rider arguably prohibits the patented inventions for approximately be a lack of expertise at the PTO. Because
use of federal funds to support research 20 years13. The public-policy justification psychedelics were criminalized for decades,
on psychedelics, so long as they remain for patents rests on the theory that the agency lacks personnel adept at
in schedule I. the right to exclude incentivizes drug evaluating novelty and non-obviousness in
Bills to eliminate the rider, in 2019 development, an expensive endeavor, made this field. To address this concern, a group
and 2021, both failed. However, as this riskier when other companies can copy called ‘Porta Sophia’ created a library of
Comment was going to print, the National an invention. Accordingly, companies existing psychedelic technologies to help
1670 Nature Medicine | VOL 27 | October 2021 | 1666–1671 | www.nature.com/naturemedicine
comment
patent applicants and PTO examiners assess A final looming issue is the question Policy, Biotechnology, and Bioethics at Harvard Law
the novelty of inventions19. of which healthcare or para-medical School, Cambridge, MA, USA. 2University of New
Other potential solutions include professionals will be empowered to help Hampshire Franklin Pierce School of Law, Concord,
encouraging inventors to sign patent patients. It is not only licensed physicians NH, USA. 3Harvard Law School, Cambridge,
pledges — promises not to enforce who are interested in psychedelics practice, MA, USA.
patent rights under certain conditions. and it remains unclear who else may play ✉e-mail: mmarks@law.harvard.edu
During the COVID-19 pandemic, some leading roles and what licensure regimes
companies took the Open COVID might look like. Published online: 4 October 2021
Pledge, promising not to enforce their One approach would center psychedelics https://doi.org/10.1038/s41591-021-01530-3
rights against competitors using their within a prescription model that requires
technologies to address the pandemic. licensed prescribers, typically physicians. References
Impressive advancements have been This model has benefits, but it may raise 1. Marks, M. N. Y. Univ. J. Legis. Public Policy 21, 69–140 (2018).
2. Davis, A. K. et al. JAMA Psychiatry 78, 481–489 (2021).
seen in the psychedelics space without challenges in a setting in which many 3. Mithoefer, M. C. et al. Psychopharmacol. 236, 2735–2745 (2019).
patents. Two leading non-profits, patients already use psychedelics, either 4. Griffiths, R. R., Johnson, M. W. & Carducci, M. A. J.
the Multidisciplinary Association for alone or with the assistance of healthcare Psychopharmacol. 30, 1181–1197 (2016).
5. Johnson, M. W., Griffiths, R. R. & Hendricks, P. S.
Psychedelic Studies and the Usona Institute, professionals or spiritual healers. A Neuropharmacol. 142, 143–166 (2018).
conduct clinical trials with psychedelics prescription model may not be the best 6. De Veen, B. T. H. Expert Rev. Neurother. 17, 203–212 (2016).
while eschewing patent rights. approach for everyone. 7. van Amsterdam, J., Opperhuizen, A. & van den Brink, W. Regul.
Toxicol. Pharmacol. 59, 423–429 (2011).
Restricting patents on psychedelics 8. Brooks, M. Medscape https://www.medscape.com/viewarticle/
may be necessary to promote their role in The Oregon model 921789 (2019).
the meaningful advancement of mental The state of Oregon is pursuing an alternative 9. Marks, M. M. Adm. Law Rev. 72, 649–718 (2020).
10. National Institutes of Health. https://grants.nih.gov/grants/policy/
healthcare. US law prohibits patents on model in which trained facilitators licensed nihgps/html5/section_4/4.2.7_promotion_or_legalization_of_
products of nature, including human genes; by the Oregon Health Authority will controlled_substances.htm (2021).
abstract ideas, such as those expressed administer psilocybin21. Clients seeking 11. McCall, B. Nat. Med. https://www.nature.com/articles/d41591-
by mathematical formulas; and natural access to ‘psilocybin services’, as they are 020-00001-5 (2020).
12. Michaels, T. I., Purdon, J., Collins, A. & Williams, M. T.
phenomena, including the laws of nature. called in Oregon, need not have a medical BMC Psychiatry 18, 245–259 (2018).
Some might also think of psychedelics diagnosis to participate. Because clinical-trial 13. Hickey, K.J. Congress. Res. Serv. https://crsreports.congress.gov/
as tools of discovery that should be free participants often report sustained feelings of product/details?prodcode=R46525 (2020).
14. US Patent 10,947,257 (filed 9 October 2018).
to all and reserved exclusively to none. wellbeing22, some believe psilocybin services 15. Angermayer, C. LinkedIn https://www.linkedin.com/pulse/
Psychiatrist Stanislav Grof once said that could help fill the current gap in preventative open-letter-tim-ferriss-value-patents-psychedelic-angermayer/
when psychedelics are used responsibly, they mental healthcare. (2021).
16. Love, S. Motherboard https://www.vice.com/en/article/m7amw4/
may do for psychiatry what the microscope The Oregon model of psilocybin services is-it-possible-to-create-an-ethical-psychedelics-company (2021).
achieved for biology and the telescope envisions the facilitator as new type of 17. Lidsky, L. STAT First Opinion https://www.statnews.com/
accomplished for astronomy20. professional trained in Western scientific 2019/07/23/patent-reform-protect-access-lifesaving-drugs/ (2019).
18. Love, S. Motherboard https://www.vice.com/en/article/g5gdzy/
knowledge as well as Indigenous uses of can-lsd-treat-food-allergies-we-dont-know-but-its-already-
Improving access and acceptance plant medicines. The Oregon Psilocybin been-patented (2021).
As the evidence base for psychedelic Advisory Board, appointed by Governor 19. Richert, L. Psychology Today https://www.psychologytoday.com/
us/blog/hygieias-workshop/202107/the-complicated-patenting-
therapies grows, it is essential that payers Kate Brown in March, is advising the our-psychedelic-future (2021).
expand coverage. Oregon Health Authority on rules for this 20. Schreck, M. MAPS Bulletin 21, 26–28 (2011).
Many who might benefit from emerging industry23. 21. Marks, M. Gizmodo https://gizmodo.com/inside-the-fight-to-
psychedelics may be on Medicaid, and Given the worsening mental-health legalize-psilocybin-therapy-in-oreg-1845450885 (2020).
22. Agin-Liebes, G. I. et al. J. Psychopharmacol. 34, 155–166 (2020).
even if private insurers begin coverage, crisis, and a lack of innovation in 23. Acker, L. The Oregonian https://www.oregonlive.com/pacific-
many patients will be unable to access these psychopharmacology, it is urgent that the northwest-news/2021/03/governor-appoints-board-to-oversee-
therapies. Coverage should therefore be US Congress make funds available for oregons-new-psychedelic-mushroom-program.html (2021).
central to policy-reform efforts in federal psychedelics research, which is currently
and state governments, or the liberalization sustained mainly by corporate and private Acknowledgements
of psychedelics may leave those most in donors. As with cannabis regulation, We are grateful for the support of the Saisei Foundation
and thank E. Wright Clayton and R. Sachs for helpful
need without access. there will be challenges and opportunities
comments on an earlier draft of this article.
Many physicians who wish to incorporate when a medical model is introduced
psychedelics into their practices need over a preexisting less-regulated model. Author contributions
training, and it will be essential to create This, however, is a good problem for the M.M. and I.G.C. drafted the manuscript.
evidence-based clinical-practice guidelines. medico-legal community to face, compared
Standards may help reduce fear among with the status quo, in which the answer is Competing interests
some healthcare professionals about medical firmly ‘just say no’. ❐ I.G.C. and M.M.’s work was supported by the Project on
malpractice liability if patients have bad Psychedelics Law and Regulation (POPLAR), which itself
Mason Marks1,2 ✉ and I. Glenn Cohen 1,3
receives funding from the Saisei Foundation, a non-profit
outcomes while using these therapies. But organization based in Austin, Texas. M.M. is a governor-
litigation may be necessary to shape the 1
Project on Psychedelics Law and Regulation appointed member of the Oregon Psilocybin Advisory
boundaries here. (POPLAR) at the Petrie-Flom Center for Health Law Board and chairs its licensing subcommittee.
Nature Medicine | VOL 27 | October 2021 | 1666–1671 | www.nature.com/naturemedicine 1671
You might also like
- Perform Final Commissioning - KCM 831 Control With KDM Drive, Monospace 500 and 700, Ecosystem MR, and TransysDocument167 pagesPerform Final Commissioning - KCM 831 Control With KDM Drive, Monospace 500 and 700, Ecosystem MR, and TransysRaja Durai100% (1)
- ServsafeDocument2 pagesServsafeapi-461183746No ratings yet
- Sexual Singularity Lenguage of LustDocument8 pagesSexual Singularity Lenguage of LustValpo Valparaiso100% (1)
- Movie Star by Lizzie Pepper - Hilary Liftin (Excerpt)Document16 pagesMovie Star by Lizzie Pepper - Hilary Liftin (Excerpt)Allen & UnwinNo ratings yet
- VIJAY 5G Front Comms Net 2021Document8 pagesVIJAY 5G Front Comms Net 2021David IbanezNo ratings yet
- Gibbs CamDocument8 pagesGibbs CamnobleadiNo ratings yet
- Blake Zucco Independent Research Period 3Document6 pagesBlake Zucco Independent Research Period 3api-321753881No ratings yet
- Machine Learning For Integrating Data in Biology and Medicine. Principles, Practice, and OpportunitiesDocument27 pagesMachine Learning For Integrating Data in Biology and Medicine. Principles, Practice, and OpportunitiesArcangelo Steelman PalmaNo ratings yet
- More Is Less: Signal Processing and The Data Deluge: SpecialsectionDocument4 pagesMore Is Less: Signal Processing and The Data Deluge: SpecialsectionGilbertNo ratings yet
- Human Genome BasicsDocument12 pagesHuman Genome BasicsPradeep Kumar. NagisettyNo ratings yet
- Status of Human DNA Research in The United States of America: A Scientometric AnalysisDocument8 pagesStatus of Human DNA Research in The United States of America: A Scientometric AnalysisMathewNo ratings yet
- Mining and Predicting Protein-Drug Interaction Network of Breast Cancer Risk GenesDocument14 pagesMining and Predicting Protein-Drug Interaction Network of Breast Cancer Risk GenesMD. Ziaul Haque ZimNo ratings yet
- Deciphering The Rhizosphere Microbiome For DiseaseDocument6 pagesDeciphering The Rhizosphere Microbiome For DiseaseRicardo Cesar Costa BachegaNo ratings yet
- ToUSE-Science-2010-Costanzo-The Genetic Landscape of A CellDocument8 pagesToUSE-Science-2010-Costanzo-The Genetic Landscape of A CellSwamy Krishna B SaiNo ratings yet
- Wu Et Al. - 2013 - Science (New York, N.Y.)Document6 pagesWu Et Al. - 2013 - Science (New York, N.Y.)Mariana NannettiNo ratings yet
- Urisu 2006Document2 pagesUrisu 2006Olavo Amorim SantosNo ratings yet
- Detecting Drug-Resistant Tuberculosis in Chest RadiographsDocument12 pagesDetecting Drug-Resistant Tuberculosis in Chest RadiographsMuhammad ReskyNo ratings yet
- 2006 Article p469Document14 pages2006 Article p469John Aeron CaluagNo ratings yet
- Bioinformatics: An Overview and Its Applications: W.J.S. Diniz and F. CanduriDocument21 pagesBioinformatics: An Overview and Its Applications: W.J.S. Diniz and F. CanduriLUIZ FELIPE ELVERNo ratings yet
- Genome, Transcriptome and Proteome PDFDocument17 pagesGenome, Transcriptome and Proteome PDFAdn CodeNo ratings yet
- Biology and Data Interpretation Techniques ConceptsDocument2 pagesBiology and Data Interpretation Techniques ConceptsVani DarjiNo ratings yet
- Computational Studies of Drug Repurposing and Synergism of Lopinavir Oseltamivir and Ritonavir Binding With SARS CoV 2 Protease Against COVID 19Document7 pagesComputational Studies of Drug Repurposing and Synergism of Lopinavir Oseltamivir and Ritonavir Binding With SARS CoV 2 Protease Against COVID 19apotekersukses88No ratings yet
- Science:, 1253 (2012) Henry J. Haiser and Peter J. TurnbaughDocument4 pagesScience:, 1253 (2012) Henry J. Haiser and Peter J. TurnbaughJing XueNo ratings yet
- Big Data in Digital Healthcare Lessons Learnt and RecommendationsDocument10 pagesBig Data in Digital Healthcare Lessons Learnt and RecommendationsFisiopatologia DxINo ratings yet
- Biology ProjectDocument18 pagesBiology Projectheyutkarsh3611No ratings yet
- Biology Project PDFDocument17 pagesBiology Project PDFheyutkarsh3611No ratings yet
- The Human Genome Project 1Document4 pagesThe Human Genome Project 1Angela NeriNo ratings yet
- Commentary: Science, Policy, and The Transparency of ValuesDocument5 pagesCommentary: Science, Policy, and The Transparency of ValuesMade DeddyNo ratings yet
- Works Cited Full BibDocument8 pagesWorks Cited Full Bibapi-606096817No ratings yet
- Theranostic Nanoparticles A Recent Breakthrough in Nanotechnology 2157 7439.1000e114Document1 pageTheranostic Nanoparticles A Recent Breakthrough in Nanotechnology 2157 7439.1000e114Demouthie EsmeraldoNo ratings yet
- PGH 25 Años DespuésDocument6 pagesPGH 25 Años DespuésLaura EscobarNo ratings yet
- Book Project CapstoneDocument4 pagesBook Project Capstoneapi-606096817No ratings yet
- J Pediatrneurol 2020 08 012Document11 pagesJ Pediatrneurol 2020 08 012succa07No ratings yet
- Probiotics, Prebiotics and The Microbiome PDFDocument10 pagesProbiotics, Prebiotics and The Microbiome PDFkuldeep sainiNo ratings yet
- 2012 122tr PHDDocument122 pages2012 122tr PHDNguyễn Tiến HồngNo ratings yet
- Knowledge Graphs and Their Applications in DrugDocument14 pagesKnowledge Graphs and Their Applications in Drugnassar.dakkouneNo ratings yet
- FDDSV 02 1082065 - RevDocument13 pagesFDDSV 02 1082065 - RevAlan TaleviNo ratings yet
- Re Produc Ibility and Research Ethics 10312016 CleanDocument13 pagesRe Produc Ibility and Research Ethics 10312016 CleanFelixNo ratings yet
- Gene CloningDocument17 pagesGene CloningOmaraye JoshuaNo ratings yet
- PDFDocument429 pagesPDFOki NurpatriaNo ratings yet
- Molecular Labs AssignmentDocument34 pagesMolecular Labs AssignmentMustafa MahmoodNo ratings yet
- Ethical Issues of CRISPR Technology and Gene Edithing Through The Lens of SolidarityDocument13 pagesEthical Issues of CRISPR Technology and Gene Edithing Through The Lens of SolidarityBadtz MaruNo ratings yet
- Ethical Issues of The Human Genome ProjectDocument7 pagesEthical Issues of The Human Genome Projectsam_max_bladerunnerNo ratings yet
- Yim 2018 Converg. Sci. Phys. Oncol. 4 014001 PDFDocument12 pagesYim 2018 Converg. Sci. Phys. Oncol. 4 014001 PDFMikael HamNo ratings yet
- Molecular Biology Dissertation IdeasDocument4 pagesMolecular Biology Dissertation IdeasBuyCollegePaperOnlineUK100% (1)
- bbw114 PDFDocument17 pagesbbw114 PDFJanescu LucianNo ratings yet
- DTMinerDocument8 pagesDTMinerwhitecommaNo ratings yet
- Provisional: Molecular Sets (MOSES) : A Benchmarking Platform For Molecular Generation ModelsDocument22 pagesProvisional: Molecular Sets (MOSES) : A Benchmarking Platform For Molecular Generation ModelsRonaldo PaxDeorumNo ratings yet
- ExosomesDocument18 pagesExosomesMahmood-S ChoudheryNo ratings yet
- Big Picture Nanomedicine PDFDocument14 pagesBig Picture Nanomedicine PDFSaraVelozNo ratings yet
- Nano Based Drug Delivery Systems: Recent Developments and Future ProspectsDocument34 pagesNano Based Drug Delivery Systems: Recent Developments and Future ProspectsshowravNo ratings yet
- Science Magazine 5696 2004-10-22Document120 pagesScience Magazine 5696 2004-10-22WillimSmithNo ratings yet
- BALLOCANAG, Jessica, BSN1i, STS JournalReadingFinalsDocument13 pagesBALLOCANAG, Jessica, BSN1i, STS JournalReadingFinalsJessica Saludar BallocanagNo ratings yet
- Occupacional BenceDocument11 pagesOccupacional Bencejlcano1210No ratings yet
- Evolution of Human Diseases - HO Smail PDFDocument17 pagesEvolution of Human Diseases - HO Smail PDFdoeditNo ratings yet
- Biomolecules 11 00090 v2Document4 pagesBiomolecules 11 00090 v2Windu KumaraNo ratings yet
- Project HUMADocument9 pagesProject HUMAOmar AlaaNo ratings yet
- Running HeadDocument19 pagesRunning HeadDANIELA FERNANDA MUÑOZ LARANo ratings yet
- Fnagi 15 1184435Document15 pagesFnagi 15 1184435maria fensterseiferNo ratings yet
- Mapping The Antigenic and Genetic Evolution of in Uenza VirusDocument7 pagesMapping The Antigenic and Genetic Evolution of in Uenza Virusaleisha97No ratings yet
- Bioinformatics Lecture NotesDocument5 pagesBioinformatics Lecture NotesChaitra A RNo ratings yet
- 5G - Scientific EvidenceDocument8 pages5G - Scientific EvidenceRosa AzulNo ratings yet
- Correspondence: Letters To The EditorDocument1 pageCorrespondence: Letters To The EditorGraciela Villarte SmithNo ratings yet
- Cancer NanotechnologyDocument6 pagesCancer NanotechnologyMirzaq D MqiiuNo ratings yet
- Ligand Efficiency Indices for Drug Discovery: Towards an Atlas-Guided ParadigmFrom EverandLigand Efficiency Indices for Drug Discovery: Towards an Atlas-Guided ParadigmNo ratings yet
- CLP-680ND PC v00Document25 pagesCLP-680ND PC v00minoltaep4050No ratings yet
- TP-N01013 Determination of Gardner Color NumberDocument2 pagesTP-N01013 Determination of Gardner Color Numberm.cj1No ratings yet
- T.galen Hieronymus - Radionic PioneerDocument17 pagesT.galen Hieronymus - Radionic Pioneeriosua100% (1)
- F1855 1479757-1Document2 pagesF1855 1479757-1Thaweekarn ChangthongNo ratings yet
- Avellanus - Fabulae Divales PDFDocument231 pagesAvellanus - Fabulae Divales PDFHakanana Fortissime100% (1)
- Loctite Ea 3478 - Carga MetalicaDocument3 pagesLoctite Ea 3478 - Carga Metalicafrancisca ferrerNo ratings yet
- Patent Assignment Cover Sheet: Electronic Version v1.1 Stylesheet Version v1.2Document42 pagesPatent Assignment Cover Sheet: Electronic Version v1.1 Stylesheet Version v1.2OneNation100% (3)
- AAKER - Measuring Brand EquityDocument19 pagesAAKER - Measuring Brand EquityAmit PandeyNo ratings yet
- Taar v. Lawan (Case Digest)Document5 pagesTaar v. Lawan (Case Digest)Dan Christian Dingcong Cagnan100% (1)
- S1 - Chapter Review 2Document5 pagesS1 - Chapter Review 2Mweene Ruth MatongoNo ratings yet
- Remedies of The TaxpayerDocument4 pagesRemedies of The TaxpayerHaze Q.No ratings yet
- Patente Sistema de Envase Rotativo 3Document38 pagesPatente Sistema de Envase Rotativo 3Valvite JuniorNo ratings yet
- Datasheet L298Document13 pagesDatasheet L298Anugera DewanggaNo ratings yet
- Solutions To Problems: 13/e, Solutions Manual (For Instructor Use Only)Document5 pagesSolutions To Problems: 13/e, Solutions Manual (For Instructor Use Only)Adam IngberNo ratings yet
- What Happened in These MonthsDocument15 pagesWhat Happened in These MonthsAliakbar Ismail KathanawalaNo ratings yet
- Agency KissaDocument14 pagesAgency KissaPaul Christopher Pineda100% (1)
- The Map To Everywhere by Carrie Ryan and John Parke DavisDocument60 pagesThe Map To Everywhere by Carrie Ryan and John Parke DavisLittle, Brown Books for Young Readers0% (1)
- Rockin' Around The Christmas Tree PDFDocument1 pageRockin' Around The Christmas Tree PDFHouston NielNo ratings yet
- GFT 87aDocument6 pagesGFT 87aRoni SocompiNo ratings yet
- Joanna Demers Steal This Music How IntelDocument4 pagesJoanna Demers Steal This Music How Intel6-16301No ratings yet
- Wordsworth PoetryDocument1 pageWordsworth PoetryFranchone BeyNo ratings yet
- Iron Kingdom Blackwood - Leafstone - and - SilkDocument5 pagesIron Kingdom Blackwood - Leafstone - and - SilkLeonarius NomadaNo ratings yet
- Practical Guide To Commercial ContractsDocument5 pagesPractical Guide To Commercial ContractsJackdan12No ratings yet
- Hex Tile Maps - Village and Roads PackDocument10 pagesHex Tile Maps - Village and Roads Packhygino vinicius100% (1)
- Cone Crusher CH Series: Rock Processing Chapter DDocument1 pageCone Crusher CH Series: Rock Processing Chapter DMortal KombatNo ratings yet