Professional Documents
Culture Documents
Sodium Metabisulfite - Wikipedia
Uploaded by
Mohamad MawadCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Sodium Metabisulfite - Wikipedia
Uploaded by
Mohamad MawadCopyright:
Available Formats
Sodium
metabisulfite
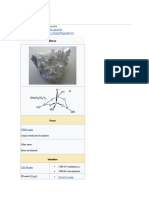
Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium
metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical
formula Na2S2O5. The substance is sometimes referred to as disodium
metabisulfite. It is used as a disinfectant, antioxidant, and preservative agent.[2]
Sodium metabisulfite
Names
Other names
Sodium pyrosulfite
Sodium disulfite
Identifiers
CAS Number 7681-57-4 (https://commonchemistry.cas.org/d
etail?cas_rn=7681-57-4)
3D model (JSmol) Interactive image (https://chemapps.stolaf.edu/
jmol/jmol.php?model=%5BO-%5DS%28%3DO%2
9S%28%3DO%29%28%3DO%29%5BO-%5D.%5B
Na%2B%5D.%5BNa%2B%5D)
ChEBI CHEBI:114786 (https://www.ebi.ac.uk/chebi/se
archId.do?chebiId=114786)
ChEMBL ChEMBL2016976 (https://www.ebi.ac.uk/chem
bldb/index.php/compound/inspect/ChEMBL20
16976)
ECHA InfoCard 100.028.794 (https://echa.europa.eu/substance
-information/-/substanceinfo/100.028.794)
EC Number 231-673-0
E number E223 (preservatives)
PubChem CID 656671 (https://pubchem.ncbi.nlm.nih.gov/com
pound/656671)
RTECS number UX8225000
UNII 4VON5FNS3C (https://fdasis.nlm.nih.gov/srs/sr
sdirect.jsp?regno=4VON5FNS3C)
CompTox Dashboard (EPA) DTXSID0029684 (https://comptox.epa.gov/das
hboard/chemical/details/DTXSID0029684)
InChI
InChI=1S/2Na.H2O5S2/c;;1-6(2)7(3,4)5/h;;(H,1,2)(H,3,4,5)/q2*+1;/p-2
SMILES
[O-]S(=O)S(=O)(=O)[O-].[Na+].[Na+]
Properties
Chemical formula Na2S2O5
Molar mass 190.107 g/mol
Appearance White to yellow powder
Odor Faint SO2
Density 1.48 g/cm3
Melting point 170 °C (338 °F; 443 K) decomposition begins at
150 °C
Solubility in water 45.1 g/100 mL (0 °C)
65.3 g/100 mL (20 °C)
81.7 g 100 mL (100 °C)
Solubility Very soluble in glycerol
Slightly soluble in ethanol
Hazards
GHS labelling:
Pictograms
Signal word Danger
Hazard statements H302, H318
Precautionary statements P264, P270, P280, P301+P312,
P305+P351+P338, P310, P330, P501
NFPA 704 (fire diamond)
0
2 1
NIOSH (US health exposure limits):
PEL (Permissible) None[1]
REL (Recommended) TWA 5 mg/m3[1]
IDLH (Immediate danger) N.D.[1]
Safety data sheet (SDS) Mallinckrodt MSDS (http://bulkpharm.mallinckr
odt.com/_attachments/msds/S4378.htm)
Related compounds
Other anions Sodium sulfite
Sodium bisulfite
Other cations Potassium metabisulfite
Related compounds Sodium dithionite
Sodium thiosulfate
Sodium sulfate
Except where otherwise noted, data are given for materials in their standard state (at
25 °C [77 °F], 100 kPa).
verify (https://en.wikipedia.org/w/index.php?title=Special:ComparePages&rev1=4488
50808&page2=Sodium+metabisulfite) (what is ?)
Infobox references
Preparation
Sodium metabisulfite can be prepared by treating a solution of sodium
hydroxide with sulfur dioxide.[3] When conducted in warm water, Na2SO3 initially
precipitates as a yellow solid. With more SO2, the solid dissolves to give the
disulfite, which crystallises upon cooling.[4]
SO2 + 2 NaOH → Na2SO3 + H2O
SO2 + Na2SO3 → Na2S2O5
which yields a residue of colourless solid Na2S2O5.
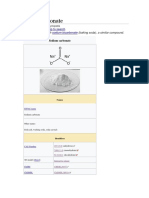
Chemical structure
The anion metabisulfite consists of an SO2 group linked to an SO3 group, with
the negative charge more localised on the SO3 end. The S–S bond length is
2.22 Å, and the "thionate" and "thionite" S–O distances are 1.46 and 1.50 Å,
respectively.[5]
Reactivity
Upon dissolution in water, bisulfite is generated:
Na2S2O5 + H2O → 2 Na+ + 2 HSO3−
Uses
Safety
References
External links
Retrieved from
"https://en.wikipedia.org/w/index.php?
title=Sodium_metabisulfite&oldid=111548
2512"
Last edited 5 days ago by 77.75.244.136
You might also like
- Hydrazine: A Colorless Flammable LiquidDocument17 pagesHydrazine: A Colorless Flammable Liquidameen alhakimiNo ratings yet
- Chelating Extractants: Equilibrium Constant of Liquid–Liquid Distribution ReactionsFrom EverandChelating Extractants: Equilibrium Constant of Liquid–Liquid Distribution ReactionsNo ratings yet
- AnilineDocument8 pagesAnilinetrianaNo ratings yet
- Gas Sweetening and Processing Field ManualFrom EverandGas Sweetening and Processing Field ManualRating: 4 out of 5 stars4/5 (7)
- 4 Nitroaniline WikipediaDocument1 page4 Nitroaniline WikipediaWenkunZhangNo ratings yet
- GC/LC, Instruments, Derivatives in Identifying Pollutants and UnknownsFrom EverandGC/LC, Instruments, Derivatives in Identifying Pollutants and UnknownsNo ratings yet
- Nitroglycerin: A History of Explosive Medical UsesDocument19 pagesNitroglycerin: A History of Explosive Medical UsesBalaji BscRTNo ratings yet
- Newer Redox Titrants: International Series of Monographs in Analytical ChemistryFrom EverandNewer Redox Titrants: International Series of Monographs in Analytical ChemistryNo ratings yet
- Potassium Permanganate - WikipediaDocument8 pagesPotassium Permanganate - WikipediaSuraj SinghNo ratings yet
- Ethylene Oxide - WikipediaDocument1 pageEthylene Oxide - WikipediathhNo ratings yet
- Acetone - WikipediaDocument65 pagesAcetone - WikipediaDylNo ratings yet
- Hydrogen SulfideDocument151 pagesHydrogen SulfidereddygrNo ratings yet
- Borax: Jump To Navigation Jump To SearchDocument4 pagesBorax: Jump To Navigation Jump To Searchelika.alfonsoNo ratings yet
- Diphenyl Ether Is The Organic CompoundDocument17 pagesDiphenyl Ether Is The Organic CompoundIslamic WorldNo ratings yet
- Sodium Chloride - WikipediaDocument53 pagesSodium Chloride - WikipediadaribeefaNo ratings yet
- MDI UsesDocument18 pagesMDI UsesYogeshNo ratings yet
- Methanol: A Colorless, Flammable Liquid With Toxic EffectsDocument76 pagesMethanol: A Colorless, Flammable Liquid With Toxic Effectsrajesh indukuriNo ratings yet
- Carbon disulfide: properties and uses of this colorless volatile liquidDocument29 pagesCarbon disulfide: properties and uses of this colorless volatile liquidꦗꦮꦶ ꦤꦸꦱꦤ꧀ꦠꦫNo ratings yet
- Sulfuric Acid - Wikipedia PDFDocument94 pagesSulfuric Acid - Wikipedia PDFrajesh indukuriNo ratings yet
- From Wikipedia, The Free Encyclopedia Sodium HydroxideDocument4 pagesFrom Wikipedia, The Free Encyclopedia Sodium Hydroxideanon_769688211No ratings yet
- ThioureaDocument8 pagesThioureaWidhy LestariNo ratings yet
- Maleic Anhydride - WikipediaDocument31 pagesMaleic Anhydride - WikipediaObaidullah ObaidiNo ratings yet
- Chemistry of Ascorbic AcidDocument6 pagesChemistry of Ascorbic Acidarieljay naunganNo ratings yet
- Salicyl Alcohol - WikipediaDocument1 pageSalicyl Alcohol - WikipediathhNo ratings yet
- Plan of ActionDocument8 pagesPlan of Actionapi-3828651No ratings yet
- Potassium Nitrate: Properties and Uses of SaltpeterDocument6 pagesPotassium Nitrate: Properties and Uses of SaltpeterLeoNo ratings yet
- ButainDocument9 pagesButainshishishi123No ratings yet
- HydrazineDocument76 pagesHydrazinenat miumNo ratings yet
- Phenyl Salicylate - WikipediaDocument5 pagesPhenyl Salicylate - WikipediaHUMANITY WORLDNo ratings yet
- 2names and Identifiers: 1.3crystal StructuresDocument8 pages2names and Identifiers: 1.3crystal Structuresrajesh_rbpNo ratings yet
- Extreme Cooling System Degreaser SDSDocument7 pagesExtreme Cooling System Degreaser SDSAsadNo ratings yet
- ResorcinolDocument9 pagesResorcinolAssassin's j :uNo ratings yet
- Sodium Carbonate FAO PDFDocument18 pagesSodium Carbonate FAO PDFAnonymous ZON7qMNo ratings yet
- Aniline: Properties, Production, Reactions, and DerivativesDocument66 pagesAniline: Properties, Production, Reactions, and DerivativesBansari PatelNo ratings yet
- LXS RCH Kleinknecht 181011 16-9 Engl - FinalDocument32 pagesLXS RCH Kleinknecht 181011 16-9 Engl - Finalrossifam777No ratings yet
- Declaration of Rohs & Pfos ConformityDocument2 pagesDeclaration of Rohs & Pfos ConformitySanthoshNo ratings yet
- Poly (Styrenesulfonic Acid-Co-Maleic Acid), Sodium Salt - Pssa-MaDocument2 pagesPoly (Styrenesulfonic Acid-Co-Maleic Acid), Sodium Salt - Pssa-MaHarita N ChamidyNo ratings yet
- Organic Peroxides Their Safe Handling and UseDocument16 pagesOrganic Peroxides Their Safe Handling and UseМаксим ХилоNo ratings yet
- Sodium bicarbonate: versatile chemical raising agent and disinfectantDocument63 pagesSodium bicarbonate: versatile chemical raising agent and disinfectantSridhar RaparthiNo ratings yet
- Product Information: MB011 EDTA Disodium Salt Dihydrate, For Molecular BiologyDocument1 pageProduct Information: MB011 EDTA Disodium Salt Dihydrate, For Molecular BiologyAbid SiddiquiNo ratings yet
- SMBS GradesDocument8 pagesSMBS GradestinuvalsapaulNo ratings yet
- NMR Solvent Data ChartDocument2 pagesNMR Solvent Data ChartBalogh Szabolcs100% (1)
- Triethyl PhosphateDocument18 pagesTriethyl PhosphateEjal MahritNo ratings yet
- Hydrated LimeDocument7 pagesHydrated LimerememberNo ratings yet
- 1712 PDF PDFDocument6 pages1712 PDF PDFYudhystira Iqbal Permana PutraNo ratings yet
- CHLORIDE TEST TABLETS EnglishDocument10 pagesCHLORIDE TEST TABLETS EnglishAmit BishtNo ratings yet
- Organic Compound Chemical Formula N H C O Amine Carbonyl Functional GroupDocument4 pagesOrganic Compound Chemical Formula N H C O Amine Carbonyl Functional GroupAshraf Un NisaNo ratings yet
- Identification of The Substance and of The Company: Date: 23/01/2012. Previous Version: 05/12/2011. Page 1/15Document15 pagesIdentification of The Substance and of The Company: Date: 23/01/2012. Previous Version: 05/12/2011. Page 1/15Pandji ZFNo ratings yet
- Material Safety Data SheetDocument7 pagesMaterial Safety Data SheetAnonymous NgcpLQiNo ratings yet
- Methyl Isocyanate - WikipediaDocument28 pagesMethyl Isocyanate - WikipediaABDulNafeNo ratings yet
- Gsyuasa SlaDocument22 pagesGsyuasa SlaAlan Stone RebeloNo ratings yet
- DatasheetDocument98 pagesDatasheetelectronistulNo ratings yet
- Iron(II) sulfate: essential iron supplementDocument8 pagesIron(II) sulfate: essential iron supplementShayan ZafarNo ratings yet
- Msds-014 Preco Hydrotard - Sds11287 - En1Document5 pagesMsds-014 Preco Hydrotard - Sds11287 - En1joker batmanNo ratings yet
- ParathionDocument6 pagesParathionAssassin's j :uNo ratings yet
- Polymerization of MonomersDocument27 pagesPolymerization of MonomersMustafa SaglamNo ratings yet
- Formaldehyde - H2CO - PubChemDocument95 pagesFormaldehyde - H2CO - PubChemRuchita PoilkarNo ratings yet
- Adipic Acid - Wikipedia PDFDocument24 pagesAdipic Acid - Wikipedia PDFKalpesh DetheNo ratings yet
- Ostra Crude Oil (70 - 30)Document1 pageOstra Crude Oil (70 - 30)coolwet90No ratings yet
- An 245 2016 Spray Drying Microalgae 0 0Document5 pagesAn 245 2016 Spray Drying Microalgae 0 0Gaston CassaroNo ratings yet
- SP 5160Document2 pagesSP 5160Dileepa DissanayakeNo ratings yet
- Safety Data Sheet for LGFP 2 Lubricating GreaseDocument6 pagesSafety Data Sheet for LGFP 2 Lubricating GreaseZumrotus Saadah AbazNo ratings yet
- An Introduction To Wax EmulsionsDocument21 pagesAn Introduction To Wax Emulsionszamalala100% (1)
- Science Class X Sample Paper Test 02 For Board Exam 2024 AnswersDocument14 pagesScience Class X Sample Paper Test 02 For Board Exam 2024 Answerssingh2008adityaNo ratings yet
- C15PS3ADocument4 pagesC15PS3ARoxanne de RoxasNo ratings yet
- Conclusion in Physics 72.1Document2 pagesConclusion in Physics 72.1Mark Charles TarrozaNo ratings yet
- Biomolecules Key Notes 2020Document14 pagesBiomolecules Key Notes 2020Pon Adityan JeyamuruganNo ratings yet
- Reformer Convection Coils Explained PT 1 X 0Document7 pagesReformer Convection Coils Explained PT 1 X 0David PierreNo ratings yet
- MS1200C Mineral Sorting For Lump CoalDocument2 pagesMS1200C Mineral Sorting For Lump CoalnataNo ratings yet
- Metallurgical Engineering Scheme of Teaching and ExamDocument12 pagesMetallurgical Engineering Scheme of Teaching and ExamSuraj KumarNo ratings yet
- PCV CableDocument12 pagesPCV CableMahamud MusaNo ratings yet
- Material Balance in Unit OperationsDocument75 pagesMaterial Balance in Unit OperationsAcademicBMNo ratings yet
- Chemistry A Molecular Approach 2nd Edition Tro Test BankDocument35 pagesChemistry A Molecular Approach 2nd Edition Tro Test Bankstrewmerils1ej3n100% (25)
- Fluorescence IntroductionDocument7 pagesFluorescence Introductionprakush_prakushNo ratings yet
- Moments of ForcesDocument24 pagesMoments of ForcesChristine Torrepenida RasimoNo ratings yet
- Thermite PDFDocument8 pagesThermite PDFPui KuanNo ratings yet
- Physics 5 SetsDocument17 pagesPhysics 5 SetsIshan SubediNo ratings yet
- Predicting Hydrocarbon Dew PointDocument12 pagesPredicting Hydrocarbon Dew PointOng SooShinNo ratings yet
- Astm D86Document27 pagesAstm D86dennise8100% (1)
- Toxicokinetic PDFDocument29 pagesToxicokinetic PDFKirush MitaNo ratings yet
- 2014 BookDocument92 pages2014 Bookbrian delgado de lucioNo ratings yet
- BIOCHEMISTRY CHAPTER 1 INTRODUCTION With CONCEPT MAPDocument4 pagesBIOCHEMISTRY CHAPTER 1 INTRODUCTION With CONCEPT MAPKASHMIR R3No ratings yet
- Excursions in Statistical Dynamics: Gavin E. CrooksDocument117 pagesExcursions in Statistical Dynamics: Gavin E. CrookspzvpzvNo ratings yet
- BookDocument44 pagesBookDr-Mandeep SinghNo ratings yet
- Effect of Water Content and Tween 80 To The Stability of Emulsified BiodieselDocument7 pagesEffect of Water Content and Tween 80 To The Stability of Emulsified BiodieselTaurusVõNo ratings yet
- SDS for Soda LimeDocument2 pagesSDS for Soda LimeRahmida FadhliaNo ratings yet
- Equi Circuit of SRR ArrayDocument4 pagesEqui Circuit of SRR ArrayJagadish BabuNo ratings yet
- Shahreza Agung AlfatihDocument4 pagesShahreza Agung AlfatihShahreza agungNo ratings yet