Professional Documents
Culture Documents
Stroke
Uploaded by
christianyecyecanOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Stroke
Uploaded by
christianyecyecanCopyright:
Available Formats
CASE REPORT
published: 12 July 2022
doi: 10.3389/fnins.2022.877479
Diabetic Striatopathy Complicated
With Acute Ischemic Stroke: A Case
Report
Xiao Huang 1† , Junli Qi 1† , Yiding Li 2 , Jianhui Li 1 and Meng-Ge Yang 1,3*
1
Department of Neurology, The Second Affiliated Hospital of Henan University of Science and Technology, Luoyang, China,
2
Department of Orthopaedic Surgery, The Second Affiliated Hospital, Henan University of Science and Technology,
Luoyang, China, 3 Department of Neurology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and
Technology, Wuhan, China
Diabetic striatopathy (DS) is a rare complication secondary to hyperglycemia, featured by
the choreiform movements and reversible striatal abnormalities on neuroimaging. Several
studies have described the clinical characteristics of DS, however, the simultaneous
occurrence of DS and acute ischemic stroke (AIS) in the striatum has not been
reported. Herein, we report a 68-year-old man with uncontrolled type 2 diabetes who
Edited by: experienced the progressive involuntary movement of the right upper and lower limbs for
Ben Nephew, 10 days. We initially considered this patient as an AIS with hemorrhage in the left basal
Worcester Polytechnic Institute,
United States
ganglia and adjacent area because his brain magnetic resonance imaging (MRI) showed
Reviewed by:
hyperintensity on fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted
Masaki Kakeyama, imaging (DWI) images, as well as slight T1-hyperintensity around T1-hypointensity.
Waseda University, Japan
However, his symptoms worsen persistently, which was inconsistent with neuroimaging
Pinaki Dutta,
Post Graduate Institute of Medical findings. Further computed tomography (CT) scan revealed an extensive hyper-density
Education and Research and focal low-density in the left striatum, suggesting the diagnosis of DS and AIS. His
(PGIMER), India
symptoms were in complete remission after 2 months of glucose control. However,
*Correspondence:
Meng-Ge Yang
striatal hyperintensity on T1 images was significantly increased compared to the initial
yangmengge0212@163.com images, which disappeared 18 months later. Additionally, DWI hyperintensity on infarction
† These authors have contributed
lesions disappeared, while softening lesions and gliosis were observed on the follow-
equally to this work up MRI images. Therefore, we finally diagnosed the patient as DS complicated with
AIS. This report highlights that DS and AIS could occur simultaneously in the striatum
Specialty section:
This article was submitted to
after hyperglycemia, which is easily misdiagnosed as AIS with hemorrhage and requires
Neuroendocrine Science, clinicians to pay more attention to avoid misdiagnosis and delayed treatment.
a section of the journal
Frontiers in Neuroscience Keywords: diabetic striatopathy, acute ischemic stroke, hyperglycemia, involuntary movement, chorea
Received: 16 February 2022
Accepted: 15 June 2022
Published: 12 July 2022
INTRODUCTION
Citation: Diabetic striatopathy (DS) is a rare complication secondary to hyperglycemia, featured by the
Huang X, Qi J, Li Y, Li J and Yang MG
choreiform movements, and reversible striatal abnormalities in neuroimaging (Chua et al., 2020).
(2022) Diabetic Striatopathy
Complicated With Acute Ischemic
In recent years, an increasing number of studies have described the clinical characteristics of
Stroke: A Case Report. DS (Kim et al., 2015; Yu et al., 2017; Wang et al., 2020; Zheng et al., 2020; Zhao et al., 2021;
Front. Neurosci. 16:877479. Dubey et al., 2022; Tsalta-Mladenov et al., 2022). However, the simultaneous occurrence of DS
doi: 10.3389/fnins.2022.877479 and acute ischemic stroke (AIS) in the striatum has not been reported. Herein, we described
Frontiers in Neuroscience | www.frontiersin.org 1 July 2022 | Volume 16 | Article 877479
Huang et al. Complications of Hyperglycemia
an unusual case who suffered from DS and AIS simultaneously TABLE 1 | Laboratory findings at the first day of hospital admission.
after hyperglycemia. We also recorded neuroimaging
Items Results Reference range
manifestations of this patient at different stages. This
report will increase our knowledge of the rare complications Age (years) 68 -
of hyperglycemia and help to rapid clinical identification Gender Male -
and intervention. Medical history type 2 diabetes -
Laboratory findings
White blood cell count, 10∧9/L 5.36 3.5–9.5
CASE PRESENTATION Neutrophil count, 10∧9/L 3.74 1.8–6.3
Lymphocyte counts, 10∧9/L 1.34 1.1–3.2
A 68-year-old Chinese man suffered from progressive
Fasting blood glucose, mmol/L 20.2 3.89–6.11
involuntary movements of the right upper and lower limbs
Hemoglobin A1c, % 14.4 4–6.5
for 10 days. Initially, his symptoms were mild and only
Total cholesterol, mmol/L 5.78 2.83–5.71
manifested as a tremor of the right limb. In the following 10 days,
LDL, mmol/L 3.6 0–41
his symptoms gradually worsened and presented with gross,
ALT, U/L 15 9–50
purposeless, irregular, rapid choreiform movements, affecting
AST, U/L 20 15–40
daily life (Supplementary Video 1). The movements were
Urea nitrogen, mmol/L 5.6 2.5–6.4
incontrollable but stopped during sleep. Physical examination
Creatinine, µmol/L 47 60–120
revealed no obvious signs of weakness in his right extremities,
Sodium, mmol/L 137.2 137–147
but for continuous choreoathetosis and slightly reduced
Potassium, mmol/L 4.77 3.5–5.3
muscle tone.
Free Triiodothyronine 3.03 3.1–6.8
This patient had type 2 diabetes for 10 years but has not
Free Thyroxine 18.05 12–22
received any hypoglycemic therapy for nearly half a year.
His fasting blood glucose was 20.2 mmol/L (normal range: Antinuclear antibody Negative Negative
3.9–6.1 mmol/L), glycosylated hemoglobin was 14.4% (normal HIV antibody Negative Negative
range: 4–6.5 mmol/L) and urine glucose was strongly positive Treponema pallidum antibody Negative Negative
(+ + ++). His urine acetone bodies were positive (+) on Urine glucose 4+ Negative
admission but turned negative after 2 days. Serum cholesterol Urine ketone 1+ Negative
was 5.78 mmol/L (normal range: 2.83–5.17 mmol/L). Other LDL, low density lipoprotein; ALT, alanine aminotransferase; AST, aspartate
laboratory test indexes, including blood routine, liver and renal aminotransferase; HIV, human immunodeficiency virus.
function, thyroid function, ceruloplasmin, serum electrolyte, and
autoimmune antibodies, were normal. No Kayser–Fleischer ring
was observed in the cornea. Additionally, this patient was not
exposed to any drugs or toxic substances. Detailed laboratory it gradually normalized and increased to a supra-normal
findings are shown in Table 1. signal (Lansberg et al., 2001; Schulz et al., 2007; Sylaja et al.,
His brain MRI showed T1-hypointensity, T2-, and 2007).
FLAIR- and DWI-hyperintensity in the left striatum and During hospitalization, insulin and statins were administrated
adjacent areas, suggesting acute cerebral infarction lesions to control the blood glucose and hyperlipemia, respectively.
(Figures 1B,C,E,F,H,I,K,L, yellow arrows). Besides, slight Aspirin was not given until discharge from the hospital
hyperintensity on apparent diffusion coefficient (ADC) images considering the potential risk of bleeding. The involuntary
(Figures 1N,O, yellow arrows) and T1-hyperintensity around movements were not improved and even worsen after
T1-hypointensity (Figures 1A,B, red arrows) were also observed. haloperidol treatment. Then, he was initiated on trihexyphenidyl
We considered these lesions as subacute infarction lesions and clonazepam after haloperidol was discontinued. His
with hemorrhage for the first time. Consistently, MRA symptom was significantly improved after 1 week and completely
images also disclosed severe atherosclerosis and stenosis of relieved at the 2-month follow-up (Supplementary video 2).
the left middle cerebral artery (MCA) (Figure 1R, green However, hyperintensity on T1-weighted MRI images within
arrow). However, he presented with progressive involuntary the striatum was increased compared to that of the initial
movements, which was inconsistent with neuroimaging images (Figures 2A,P–R, red arrows). Given the improvement
findings. Therefore, brain CT was performed and showed of symptoms and potential drug side effects, trihexyphenidyl
an extensive hyper-density (Figures 1S,T, red arrows) and and clonazepam were gradually withdrawn. During the
focal low-density (Figures 1T,U, yellow arrows). Combined next 18 months of follow-up, the patient’s blood glucose
with the patient’s clinical data and neuroimaging findings, we was well-controlled and involuntary movements did not
thought that diabetes-related striatum lesions were responsible relapse. Moreover, a re-examination of brain MRI showed
for clinical attack and progress. The infarction lesions may that the striatal hyperintensity on T1 images has disappeared
occur early after onset, or this patient suffered from the (Figure 3A) and atrophy of the left caudate nucleus with
subclinical ischemic event before onset. Because ADC images resultant dilatation of the frontal horn of the left lateral ventricle
usually showed a low signal within 1 week after a stroke, was noted (Figures 3B,E,H,K, red arrows). Additionally,
Frontiers in Neuroscience | www.frontiersin.org 2 July 2022 | Volume 16 | Article 877479
Huang et al. Complications of Hyperglycemia
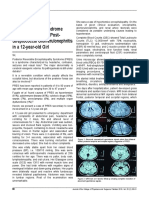
FIGURE 1 | Computed tomography (CT) and MRI of brain at admission. Diabetic striatopathy (DS)-associated striatal abnormalities were marked by red arrows, while
infarction focuses were marked by yellow arrows. Typical imaging manifestations of DS were found in the left striatum with T1 hyperintensity [(A,B), coronal images;
(P,Q), sagittal images] on MRI and hyper-density on CT images (S,T). The hypointensity on T2 (D,E), fluid-attenuation inversion recovery (FLAIR) (G,H), and apparent
diffusion coefficient (ADC) (M,N) images, as well as equal signal intensity on DWI images (J,K) were also observed in DS-associated striatal lesions. Subacute cerebral
infarctions showed T1 hypointensity [(B,C) coronal images; (Q) sagittal image], T2 hyperintensity (E,F), FLAIR hyperintensity (H,I), DWI hyperintensity (K,L), and slight
ADC hyperintensity (N,O) on MRI images, as well as hypo-density on CT images (T,U). MRA images disclosed obvious stenosis of the left middle cerebral artery [(R),
green arrow]. There were several artifacts on ADC images [(J,K), blue arrows].
DWI hyperintensity on infarction lesions disappeared on the prominently occurs in Asian women, particularly these patients
follow-up MRI (Figures 2K,L,3K,L) while ADC hyperintensity with newly diagnosed or poorly controlled diabetes. Although
increased (Figures 2N,O). Abnormal T1 hypointensity, T2 most patients with DS were negative for ketone bodies, some
hyperintensity, and FLAIR hypointensity on the infarction core, patients still suffered from episodes of ketotic hyperglycemia (Tan
as well as FLAIR hyperintensity around the infarction core, et al., 2014; Markowska et al., 2021).
suggested the formation of softening lesions and secondary
gliosis (Figures 2B–I, 3B–I, yellow arrows). Therefore, we finally
diagnosed the patient with DS and AIS. Imaging Features
Striatal hyper-density on CT images and hyperintensity on
T1-weighted MRI images are the representative neuroimaging
DISCUSSION findings (Chua et al., 2020). However, in the present case, the
striatal anomalies on the initial CT and MRI images were not
Diabetic striatopathy (DS) is a rare complication of diabetes exactly consistent, which may be attributed to the different
mellitus (DM), characterized by a prominently increased blood sensitivity (Lin et al., 2001). MRI seemed to be more sensitive;
glucose level, unilateral striatal hyper-density on CT, and furthermore, striatal hyperintensity on MRI images tended to
hyperintensity on T1-weighted MRI, as well as contralateral last longer than that on CT images (Lin et al., 2001; Chua et al.,
choreiform movement (Dong and Zhang, 2021). Bilateral striatal 2020). The striatal hyperintensity on T1-weighted images may
lesions were rarely (9.7%) seen in patients with DS, which achieve its maximum value on an average of 3 months, and
help distinguish DS from metabolic disorders, infectious, drugs, hyperintensity began to decrease thereafter (Chua et al., 2020).
or toxics-induced striatal lesions that prominently affect the This finding explained why an increased hyperintensity on T1
bilateral striatum (Chua et al., 2020; Dong and Zhang, 2021). DS images was observed on the follow-up MRI, although the clinical
Frontiers in Neuroscience | www.frontiersin.org 3 July 2022 | Volume 16 | Article 877479
Huang et al. Complications of Hyperglycemia
FIGURE 2 | Follow-up magnetic resonance imaging (MRI) of brain 2 months after onset. Diabetic striatopathy (DS)-associated striatal abnormalities were marked by
red arrows, while infarction focuses were marked by yellow arrows. DS-associated T1 hyperintensity on the left striatum was obviously extended [(A), coronal image;
(P,Q) (R): sagittal images). DS-associated T2 and FLAIR hypointensity changed to slight hyperintensity (D,G). The left striatum showed an equal signal intensity on
DWI images (J). DS-associated initial ADC hypointensity changed to equal signal intensity (M). DWI hyperintensity on infarction lesions disappeared (K,L) and ADC
hyperintensity increased (N,O). Abnormal T1 hypointensity (B,C), T2 hyperintensity (E,F), and FLAIR hypointensity on the infarction core (H,I), as well as FLAIR
hyperintensity around the infarction core (H,I) suggested the formation of softening lesions and secondary gliosis.
FIGURE 3 | Follow-up magnetic resonance imaging (MRI) of brain 18 months after onset. Diabetic striatopathy (DS)-associated striatal hyperintensity on T1 and T2
and fluid-attenuation inversion recovery (FLAIR) images were disappeared (A,D,G,J), but atrophy of the left caudate nucleus with resultant dilatation of frontal horn of
left lateral ventricle was noted [(B,E,H,K) red arrows]. Remote cerebral infarctions were observed on T1, T2, FLAIR and diffusion weighted imaging (DWI) images
[(B,C,E,F,H,I,K,L), yellow arrows].
symptoms have been completely relieved. The delayed resolution there was hypointensity on T2, FLAIR, ADC images, and equal
of T1 hyperintensity indicated that MRI may be a good tool for signal intensity on DWI images (Figures 1D,E,G,H,M,N,J,K, red
evaluating the presence of DS (Lin et al., 2001; Kitagawa et al., arrows), which were gradually increased and presented with
2017; Zheng et al., 2020). slight hyperintensity on T2 and FLAIR images (Figures 2D,G,
In addition to representative T1-weighted and CT images, we red arrows). Initial ADC hypointensity changed to equal
also recorded the dynamic changes of T2, FLAIR, DWI, and signal intensity (Figure 2M, red arrows). Abnormal striatal
ADC images of patients with DS. In the early stages of onset, signals finally disappeared (Figures 3A,D,G,J). Noticeably, the
Frontiers in Neuroscience | www.frontiersin.org 4 July 2022 | Volume 16 | Article 877479
Huang et al. Complications of Hyperglycemia
normalization of abnormal ADC signals was accompanied by vascular stenosis by a series of pathophysiological mechanisms,
a significant improvement in clinical symptoms, which was including increased plasma osmolality and hyperviscosity,
coherent with previous reports (Chu et al., 2002; Zheng et al., and also damaged the blood-cerebrospinal fluid barrier and
2020). ADC sequence has been thought to be a tool to predict the impairment of vascular fibrinolysis (Hafez et al., 2014; Pasquel
prognosis of patients with DS (Chu et al., 2002; Zaitout, 2012). et al., 2020; Jiang et al., 2021). These processes may aggravate
Although the imaging presentations of DS are generally the DS-associated striatum damage at the same time, leading
reversible in most patients, irreversible striatum lesions, such as to progressive involuntary movement. Furthermore, it is
caudate atrophy, have been occasionally reported in previous reasonable to postulate that the occurrence of acute cerebral
reports (Lucassen et al., 2017; Chatterjee et al., 2022). Long- infarction exacerbates striatum damage, and eventually leads to
term uncontrolled hyperglycemia is a major cause of irreversible atrophy of the left caudate nucleus. The presence of caudate
striatal damage (Lucassen et al., 2017; Chatterjee et al., 2022). In atrophy and infarction focuses provides more evidence for the
this case, the imaging findings of the patient were only partially microangiopathic lesion hypothesis.
reversible. Caudate atrophy might present as a severe endpoint of
DS, but it has rarely been reported in previous studies, possibly Treatment
due to timely correction of hyperglycemia or lack of long-term Hypoglycemic therapies are essential for symptom control;
follow-up in most cases (Lucassen et al., 2017). however, most patients need additional anti-chorea medications,
Lacunar infarction in the striatum might be found in brain including haloperidol, clonazepam, risperidone, trihexyphenidyl,
specimens from patients with DS, whereas imaging abnormalities and tetrabenazine (Abe et al., 2009; Chua et al., 2020; Zheng
indicative of lacunar infarction are often absent (Ohara et al., et al., 2020). When DS and AIS occur simultaneously, antiplatelet
2001; Abe et al., 2009). Imaging manifestations of DS and AIS therapy or reperfusion therapy may be beneficial, but the safety
occurring simultaneously in the striatum are easily misdiagnosed needs to be fully assessed due to the presence of microvascular
as AIS with hemorrhage, leading to delayed diagnosis and hemorrhage (Chua et al., 2020).
treatment. Dynamic imaging changes and detailed clinical data
will help us distinguish DS from AIS with hemorrhage. However,
it is still an enigma why a systemic metabolic disturbance leads Prognosis
to a unilateral symptom and why the striatum is particularly Patients with DS usually have a good prognosis with complete
vulnerable to hyperglycemia. amelioration after correction of the hyperglycemia. A few
patients developed persistent chorea even under a backdrop
Pathophysiological Mechanism of well-controlled diabetes mellitus or complete resolution of
Although the pathophysiological mechanism is still abnormal striatum signals, suggesting the presence of irreversible
unclear, several hypotheses have been proposed, including damage (Ahlskog et al., 2001; Wu et al., 2014; Roy et al.,
microangiopathic lesions, metabolic disorders, autoimmune 2016; Lucassen et al., 2017; Chatterjee et al., 2022). Severe or
inflammatory response, neurodegeneration, dopamine, and uncontrolled hyperglycemia is associated with chorea relapse and
estrogen involvement (Chang et al., 2010; Kumar Vadi et al., the attacks of acute cerebrovascular events, such as acute cerebral
2020; Zheng et al., 2020). The microangiopathic lesion hypothesis infarction and hemorrhage, leading to a poor prognosis (Carrion
has been widely known and microvascular hemorrhage seems and Carrion, 2013; Lucassen et al., 2017; Chua et al., 2020; Dong
to play a critical role in the pathogenesis of DS (Abe et al., and Zhang, 2021). Carrion et. al reported a patient who suffered
2009; Chua et al., 2020). On the one hand, hyper-density on CT from a large cerebral infarction 2 weeks after DS onset due to
and hyperintensity on MRI strongly indicate the presence of non-compliance with diabetic therapy (Carrion and Carrion,
hemorrhage and methemoglobin (Altafullah et al., 1990; Rindler 2013), suggesting active hypoglycemic therapy can effectively
et al., 2020). On the other hand, microhemorrhages are the most avoid disease progression.
common pathological findings (Chua et al., 2020), featured by
the presence of macrophages containing hemosiderin granules
(Ohara et al., 2001), focal microhemorrhage (Nath et al., 2006;
CONCLUSION
Mestre et al., 2007), hemosiderin deposition, and red blood Patients with diabetes who present with the choreiform
cell extravasation (Nath et al., 2006; Mestre et al., 2007). More movements should consider the diagnosis of DS, especially
recently, the abnormal signal on susceptibility-weighted imaging in those with poor blood sugar control. DS and AIS could
was observed in the DS-associated striatum lesions, confirming occur simultaneously after hyperglycemia, which requires
the view of microhemorrhages (Tencer and Yum, 2021). clinicians to pay more attention to avoid misdiagnosis and
Recent lacunar infarctions with reactive astrocytosis were also delayed treatment.
frequent in the damaged striatum at autopsy (Ohara et al., 2001;
Abe et al., 2009), which may be closely related to the hyaline
degeneration, stenosis, and occlusion of the lenticulostriate DATA AVAILABILITY STATEMENT
arteries (Lin et al., 2001; Nath et al., 2006). In our case, chronic
microvascular lesions in the striatum may be the common The original contributions presented in the study are included
pathological mechanism of AIS and DS. Besides, the acute in the article/Supplementary Material, further inquiries can be
ischemic attack may be induced by hyperglycemia based on directed to the corresponding author.
Frontiers in Neuroscience | www.frontiersin.org 5 July 2022 | Volume 16 | Article 877479
Huang et al. Complications of Hyperglycemia
ETHICS STATEMENT contributed to the acquisition and analysis of data. All authors
contributed to manuscript revision, read, and approved the
The studies involving human participants were reviewed submitted version.
and approved by the Medical Ethics Committee of the
Second Affiliated Hospital of Henan University of Science and FUNDING
Technology. The patients/participants provided their written
informed consent to participate in this study. Written informed This work was supported by the Medical Science and
consent was obtained from the individual(s) for the publication Technology Project of Henan Province (Grant Number:
of any potentially identifiable images or data included in LHGJ20191241).
this article.
SUPPLEMENTARY MATERIAL
AUTHOR CONTRIBUTIONS
The Supplementary Material for this article can be found
MY and XH contributed to the conception and design of the online at: https://www.frontiersin.org/articles/10.3389/fnins.
study and wrote the first draft of the manuscript. JQ, YL, and JL 2022.877479/full#supplementary-material
REFERENCES Kitagawa, M., Yamanaka, Y., Adachi, T., Ito, J., Fukase, K., Ohta,
I., et al. (2017). Diabetic Hemichorea-hemiballism after Prompt
Abe, Y., Yamamoto, T., Soeda, T., Kumagai, T., Tanno, Y., Kubo, J., et al. (2009). Improvement in Hyperglycemia. Int. Med. (Tokyo, Japan). 56, 3073–3076.
Diabetic striatal disease: clinical presentation, neuroimaging, and pathology. doi: 10.2169/internalmedicine.8615-16
Intern. Med. 48, 1135–1141. doi: 10.2169/internalmedicine.48.1996 Kumar Vadi, S., Mehta, S., Kumar, R., Tandyala, N., Singh, H., Mittal, B. R.,
Ahlskog, J. E., Nishino, H., Evidente, V. G., Tulloch, J. W., Forbes, G. S., Caviness, J. et al. (2020). Severe contralateral striatal hypometabolism in a case of diabetic
N., et al. (2001). Persistent chorea triggered by hyperglycemic crisis in diabetics. nonketotic hyperglycemic hemichorea on 18F-FDG PET/CT brain. Clin. Nucl.
Mov. Disord. 16, 890–898. doi: 10.1002/mds.1171 Med. 45, e117–e9. doi: 10.1097/RLU.0000000000002844
Altafullah, I., Pascual-Leone, A., Duvall, K., Anderson, D. C., and Taylor, S. (1990). Lansberg, M. G., Thijs, V. N., O’Brien, M. W., Ali, J. O., de Crespigny, A. J.,
Putaminal hemorrhage accompanied by hemichorea-hemiballism. Stroke. 21, Tong, D. C., et al. (2001). Evolution of apparent diffusion coefficient, diffusion-
1093–1094. doi: 10.1161/01.STR.21.7.1093 weighted, and T2-weighted signal intensity of acute stroke. AJNR Am. J.
Carrion, D. M., and Carrion, A. F. (2013). Non-ketotic hyperglycaemia Neuroradiol. 22, 637–644.
hemichorea-hemiballismus and acute ischaemic stroke. BMJ Case Rep. 2013, Lin, J. J., Lin, G. Y., Shih, C., and Shen, W. C. (2001). Presentation of striatal
bcr2012008359. doi: 10.1136/bcr-2012-008359 hyperintensity on T1-weighted MRI in patients with hemiballism-hemichorea
Chang, K. H., Tsou, J. C., Chen, S. T., Ro, L. S., Lyu, R. K., Chang, H. S., et al. caused by non-ketotic hyperglycemia: report of seven new cases and a review
(2010). Temporal features of magnetic resonance imaging and spectroscopy in of literature. J. Neurol. 248, 750–755. doi: 10.1007/s004150170089
non-ketotic hyperglycemic chorea-ballism patients. Eur. J. Neurol. 17, 589–593. Lucassen, E. B., Delfyett, W. T., and Stahl, M. C. (2017). Persistent hemichorea
doi: 10.1111/j.1468-1331.2009.02867.x and caudate atrophy in untreated diabetic striatopathy: a case report. Case Rep.
Chatterjee, S., Ghosh, R., Ojha, U. K., Diksha, Biswas, P., Benito-Leon, J., et al. Neurol. 9, 299–303. doi: 10.1159/000484201
(2022). Recurrent facial focal seizures with chronic striatopathy and caudate Markowska, K., Koziorowska-Gawron, E., Papier, P., Koszewicz, M., Budrewicz,
atrophy-a double whammy in an elderly woman with diabetes mellitus. S., Bladowska, J., et al. (2021). Easily missed or misinterpreted: diabetic
Neurohospitalist. 12, 147–150. doi: 10.1177/19418744211035370 striatopathy in the course of ketotic hyperglycaemia. Postgrad. Med. J. 97,
Chu, K., Kang, D. W., Kim, D. E., Park, S. H., and Roh, J. K. (2002). Diffusion- 539–540. doi: 10.1136/postgradmedj-2020-139499
weighted and gradient echo magnetic resonance findings of hemichorea- Mestre, T. A., Ferreira, J. J., and Pimentel, J. (2007). Putaminal petechial
hemiballismus associated with diabetic hyperglycemia: a hyperviscosity haemorrhage as the cause of non-ketotic hyperglycaemic chorea: a
syndrome? Arch. Neurol. 59, 448–452. doi: 10.1001/archneur.59.3.448 neuropathological case correlated with MRI findings. J. Neurol. Neurosurg.
Chua, C. B., Sun, C. K., Hsu, C. W., Tai, Y. C., Liang, C. Y., Tsai, I. T., et al. (2020). Psychiatr. 78, 549–550. doi: 10.1136/jnnp.2006.105387
“Diabetic striatopathy”: clinical presentations, controversy, pathogenesis, Nath, J., Jambhekar, K., Rao, C., and Armitano, E. (2006). Radiological and
treatments, and outcomes. Sci Rep. 10, 1594. doi: 10.1038/s41598-020-58555-w pathological changes in hemiballism-hemichorea with striatal hyperintensity.
Dong, M., Zhang, E. J. Y L, Teng, W, and Tian, L.. (2021). Non- J. Magn. Reson. Imaging. 23, 564–568. doi: 10.1002/jmri.20548
ketotic hyperglycemia chorea-ballismus and intracerebral hemorrhage: Ohara, S., Nakagawa, S., Tabata, K., and Hashimoto, T. (2001). Hemiballism with
a case report and literature review. Front. Neurosci. 15, 690761. hyperglycemia and striatal T1-MRI hyperintensity: an autopsy report. Mov.
doi: 10.3389/fnins.2021.690761 Disord. 16, 521–525. doi: 10.1002/mds.1110
Dubey, S., Chatterjee, S., Ghosh, R., Louis, E. D., Hazra, A., Sengupta, S., et al. Pasquel, F. J., Tsegka, K., Wang, H., Cardona, S., Galindo, R. J., Fayfman, M.,
(2022). Acute onset movement disorders in diabetes mellitus: a clinical series of et al. (2020). Clinical outcomes in patients with isolated or combined diabetic
59 patients. Eur. J. Neurol. doi: 10.1111/ene.15353 ketoacidosis and hyperosmolar hyperglycemic state: a retrospective, hospital-
Hafez, S., Coucha, M., Bruno, A., Fagan, S. C., and Ergul, A. (2014). Hyperglycemia, based cohort study. Diabetes Care. 43, 349–357. doi: 10.2337/dc19-1168
acute ischemic stroke, and thrombolytic therapy. Transl. Stroke Res. 5, 442–453. Rindler, R. S., Allen, J. W., Barrow, J. W., Pradilla, G., and Barrow, D. L.
doi: 10.1007/s12975-014-0336-z (2020). Neuroimaging of intracerebral hemorrhage. Neurosurgery. 86, E414–
Jiang, Y., Liu, N., Han, J., Li, Y., Spencer, P., Vodovoz, S. J., et al. E23. doi: 10.1093/neuros/nyaa029
(2021). Diabetes mellitus/poststroke hyperglycemia: a detrimental factor Roy, U., Das, S. K., Mukherjee, A., Biswas, D., Pan, K., Biswas, A., et al. (2016).
for tpa thrombolytic stroke therapy. Transl. Stroke Res. 12, 416–427. Irreversible hemichorea-hemiballism in a case of nonketotic hyperglycemia
doi: 10.1007/s12975-020-00872-3 presenting as the initial manifestation of diabetes mellitus. Tremor Other
Kim, Y. J., Kim, S. J., Kim, J., Kim, M.-., J., Yoon, H., et al. (2015). Chorea due to Hyperkinet Mov. (N Y). 6, 393. doi: 10.5334/tohm.301
diabetic hyperglycemia and uremia: distinct clinical and imaging features. Mov. Schulz, U. G. R., Flossmann, E., Francis, J. M., Redgrave, J. N., and Rothwell, P. M.
Disord. 30, 419–422. doi: 10.1002/mds.26148 (2007). Evolution of the diffusion-weighted signal and the apparent diffusion
Frontiers in Neuroscience | www.frontiersin.org 6 July 2022 | Volume 16 | Article 877479
Huang et al. Complications of Hyperglycemia
coefficient in the late phase after minor stroke: a follow-up study. J. Neurol. (HC-HB). Neuroradiology. 54, 1119–1120. doi: 10.1007/s00234-012-10
254, 375–383. doi: 10.1007/s00415-006-0381-y 21-0
Sylaja, P. N., Coutts, S. B., Subramaniam, S., Hill, M. D., Eliasziw, M., Zhao, S., Wu, S., Feng, L., Ni, M., Ma, M., Zhang, H., et al. (2021).
Demchuk, A. M., et al. (2007). Acute ischemic lesions of varying ages Hemichorea induced by non-ketotic hyperglycemia evaluated with F-FDG
predict risk of ischemic events in stroke/TIA patients. Neurology. 68, 415–419. and C-CFT PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 48, 3001–3002.
doi: 10.1212/01.wnl.0000252938.76188.52 doi: 10.1007/s00259-021-05240-3
Tan, Y., Xin, X., Xiao, Q., Chen, S., Cao, L., Tang, H., et al. (2014). Zheng, W., Chen, L., Chen, J. H., Lin, X., Tang, Y., Lin, X. J., et al. (2020).
Hemiballism-hemichorea induced by ketotic hyperglycemia: case report Hemichorea associated with non-ketotic hyperglycemia: a case report and
with PET study and review of the literature. Transl. Neurodegener. 3, 14. literature review. Front. Neurol. 11, 96. doi: 10.3389/fneur.2020.00096
doi: 10.1186/2047-9158-3-14
Tencer, J., and Yum, S. (2021). Teaching neuroimages: basal ganglia T1 Conflict of Interest: The authors declare that the research was conducted in the
hyperintensity and SWI signal diabetic striatopathy in an 18-year-old man with absence of any commercial or financial relationships that could be construed as a
type 1 diabetes mellitus. Neurology. doi: 10.1212/WNL.0000000000012489 potential conflict of interest.
Tsalta-Mladenov, M. E., Georgieva, D. K., and Andonova, S. P. (2022).
Hyperglycemic hemichorea due to diabetic striatopathy: case-based review. Publisher’s Note: All claims expressed in this article are solely those of the authors
Curr. Med. Res. Opin. 38, 365–369. doi: 10.1080/03007995.2021.2015159 and do not necessarily represent those of their affiliated organizations, or those of
Wang, W., Tang, X., Feng, H., Sun, F., Liu, L., Rajah, G. B., et al. (2020). Clinical
the publisher, the editors and the reviewers. Any product that may be evaluated in
manifestation of non-ketotic hyperglycemia chorea: a case report and literature
this article, or claim that may be made by its manufacturer, is not guaranteed or
review. Medicine (Baltimore). 99, e19801. doi: 10.1097/MD.0000000000019801
Wu, M. N., Ruge, D., Tsai, C. L., Hsu, C. Y., Lai, C. L., Liou, L. M., et al. endorsed by the publisher.
(2014). Periodic lateralized epileptiform discharges associated with irreversible
hyperglycemic hemichorea-hemiballism. Clin. EEG Neurosci. 45, 315–317. Copyright © 2022 Huang, Qi, Li, Li and Yang. This is an open-access article
doi: 10.1177/1550059413508555 distributed under the terms of the Creative Commons Attribution License (CC BY).
Yu, F., Steven, A., Birnbaum, L., and Altmeyer, W. (2017). T2∗ -based MR imaging The use, distribution or reproduction in other forums is permitted, provided the
of hyperglycemia-induced hemichorea-hemiballism. J. Neuroradiol. 44, 24–30. original author(s) and the copyright owner(s) are credited and that the original
doi: 10.1016/j.neurad.2016.09.005 publication in this journal is cited, in accordance with accepted academic practice.
Zaitout, Z. C. T. (2012). and MRI findings in the basal ganglia in non- No use, distribution or reproduction is permitted which does not comply with these
ketotic hyperglycaemia associated hemichorea and hemi-ballismus terms.
Frontiers in Neuroscience | www.frontiersin.org 7 July 2022 | Volume 16 | Article 877479
You might also like
- Medicine: Refractory Hyponatremia in Neuromyelitis Optica in A Pediatric PatientDocument4 pagesMedicine: Refractory Hyponatremia in Neuromyelitis Optica in A Pediatric PatientMesan KoNo ratings yet
- Association of Chronic Kidney Disease With Small Vessel Disease in Patients With Hypertensive Intracerebral HemorrhageDocument7 pagesAssociation of Chronic Kidney Disease With Small Vessel Disease in Patients With Hypertensive Intracerebral HemorrhageSofia ImaculataNo ratings yet
- A Case of A Young Man With Severe HypertensiDocument4 pagesA Case of A Young Man With Severe HypertensiProject dronacharyapwNo ratings yet
- The Hyperacute Vestibular Syndrome Ear or BrainDocument2 pagesThe Hyperacute Vestibular Syndrome Ear or BrainDea CantikNo ratings yet
- Medicine: Hemichorea After Hyperglycemia CorrectionDocument3 pagesMedicine: Hemichorea After Hyperglycemia CorrectionanankastikNo ratings yet
- Ischemic Cerebral Stroke Case Report, Complications and Associated FactorsDocument7 pagesIschemic Cerebral Stroke Case Report, Complications and Associated FactorsAlfa DexterNo ratings yet
- Ischemic Cerebral Stroke Case Report, Complications and Associated FactorsDocument5 pagesIschemic Cerebral Stroke Case Report, Complications and Associated Factorsfaradilla wiyandaNo ratings yet
- Fnagi 14 800617Document10 pagesFnagi 14 800617Snezana MihajlovicNo ratings yet
- Brain and Behavior - 2020 - Feng - Reduced Thiamine Is A Predictor For Cognitive Impairment of Cerebral InfarctionDocument8 pagesBrain and Behavior - 2020 - Feng - Reduced Thiamine Is A Predictor For Cognitive Impairment of Cerebral InfarctionJasna BuhariNo ratings yet
- Hypomagnesemia and Prolonged Hospital Stay: A Case Report and Literature ReviewDocument6 pagesHypomagnesemia and Prolonged Hospital Stay: A Case Report and Literature ReviewIJAR JOURNALNo ratings yet
- Fulltext - Ams v5 Id1040Document4 pagesFulltext - Ams v5 Id1040yaneemayNo ratings yet
- AtaxiaDocument5 pagesAtaxiaMarco Antonio KoffNo ratings yet
- Case Report: Histiocytic Necrotizing Lymphadenitis (Kikuchi-Fujimoto Disease) Concurrent With Aseptic MeningitisDocument7 pagesCase Report: Histiocytic Necrotizing Lymphadenitis (Kikuchi-Fujimoto Disease) Concurrent With Aseptic MeningitisFaril RahmaNo ratings yet
- Causes of Syncope in Patients With AlzheDocument8 pagesCauses of Syncope in Patients With AlzheMichael VavosaNo ratings yet
- 58 - 18015erdheim ChesterDocument5 pages58 - 18015erdheim ChesterGerman HaroNo ratings yet
- KauabajkaDocument7 pagesKauabajkarumasadraunaNo ratings yet
- (1-3) Hypoglycemia Induced Hemichorea (HpogC) (IJGN) - Shashi PrakashDocument3 pages(1-3) Hypoglycemia Induced Hemichorea (HpogC) (IJGN) - Shashi Prakashneeraj2811No ratings yet
- Pengertian PersalinanDocument7 pagesPengertian PersalinanVicky SaputraNo ratings yet
- The Paradoxical Protective Effect of Liver Steatosis On Severity and Functional Outcome of Ischemic StrokeDocument7 pagesThe Paradoxical Protective Effect of Liver Steatosis On Severity and Functional Outcome of Ischemic StrokewinnerfromparisNo ratings yet
- JCM 11 06258Document13 pagesJCM 11 06258deja234No ratings yet
- Hydroxychloroquine-Induced Cardiomyopathy in A Patient With Limited Cutaneous Systemic SclerosisDocument3 pagesHydroxychloroquine-Induced Cardiomyopathy in A Patient With Limited Cutaneous Systemic SclerosisIngrid KoutioNo ratings yet
- TADocument7 pagesTAAngela Villanueva SocolaNo ratings yet
- Article CientificDocument3 pagesArticle CientificGrupo 4 CardiologiaNo ratings yet
- Acute Meningitis Complicated by Transverse Myelitis: Case ReportDocument2 pagesAcute Meningitis Complicated by Transverse Myelitis: Case ReportSeptian TheaNo ratings yet
- MELAS Case ReportDocument12 pagesMELAS Case ReportIRENA GENINo ratings yet
- Algoritmo Diagnostico de La Ceguera Monocular en Una Paciente Con NeurosarcoidosisDocument8 pagesAlgoritmo Diagnostico de La Ceguera Monocular en Una Paciente Con NeurosarcoidosisFarid Santiago Abedrabbo LombeydaNo ratings yet
- Fneur 11 00071Document10 pagesFneur 11 00071Nurvia AndrianiNo ratings yet
- YessDocument5 pagesYessalingh98No ratings yet
- Version of Record:: ManuscriptDocument7 pagesVersion of Record:: ManuscriptFlavia ChNo ratings yet
- Anesthetic Management For Emergency SC and Craniotomy TogetherDocument4 pagesAnesthetic Management For Emergency SC and Craniotomy TogetherOvi Octavia KaramoyNo ratings yet
- Fenoy 2017Document7 pagesFenoy 2017syarifaNo ratings yet
- Bilateral Catarct in A Teenage Firl With Type 1 Diabetes (TD1) Case ReportDocument7 pagesBilateral Catarct in A Teenage Firl With Type 1 Diabetes (TD1) Case ReportNur Asri AinunNo ratings yet
- Posterior Reversible Encephalopathy Syndrome Secondary To Acute Post-Streptococcal Glomerulonephritis in A 12-Year-Old GirlDocument2 pagesPosterior Reversible Encephalopathy Syndrome Secondary To Acute Post-Streptococcal Glomerulonephritis in A 12-Year-Old GirlwawaningNo ratings yet
- Dengue Myocarditis: Case ReportDocument3 pagesDengue Myocarditis: Case Reportallfanz_krenNo ratings yet
- Medicine: Rhabdomyolysis Following Status Epilepticus With HyperuricemiaDocument3 pagesMedicine: Rhabdomyolysis Following Status Epilepticus With HyperuricemiaIsmi RachmanNo ratings yet
- Basal Gangila CahngesDocument12 pagesBasal Gangila CahngesJakkani RavikanthNo ratings yet
- Adults 5Document5 pagesAdults 5Bshara SleemNo ratings yet
- The Relationship Between Acute Coronary Syndrome and Stress HyperglycemiaDocument6 pagesThe Relationship Between Acute Coronary Syndrome and Stress HyperglycemiahamzahNo ratings yet
- Ejbps 11145Document4 pagesEjbps 11145dralourdesreyespediatraNo ratings yet
- Gota NEJMDocument11 pagesGota NEJMPerejiloNo ratings yet
- Research On Post Reversible Encephalopathy Due To Blood TransfusionsDocument7 pagesResearch On Post Reversible Encephalopathy Due To Blood TransfusionsAnkit AgarwalNo ratings yet
- Higher Prevalence of Diabetes in Pontine Infarction Than inDocument8 pagesHigher Prevalence of Diabetes in Pontine Infarction Than inMarshall ThompsonNo ratings yet
- MMD EpidDocument10 pagesMMD EpidGilbert Sterling OctaviusNo ratings yet
- Conclusion: Long-Term Follow-Up ForDocument31 pagesConclusion: Long-Term Follow-Up ForHima HuNo ratings yet
- DIAB10.1002@ccd.28194 4Document9 pagesDIAB10.1002@ccd.28194 4marsim92No ratings yet
- Manuscript v5Document27 pagesManuscript v5Diego LowensteinNo ratings yet
- 2525-Main Article Text (Blinded Article File) - 5250-2!10!20201009Document3 pages2525-Main Article Text (Blinded Article File) - 5250-2!10!20201009FihzanNo ratings yet
- Agmr 21 0030 2Document4 pagesAgmr 21 0030 2rodribeatsNo ratings yet
- Kawasaki Current Guidelines ComparisonDocument14 pagesKawasaki Current Guidelines ComparisonRonald FernandezNo ratings yet
- A Case of Idiopathic Intracranial Hypertension Due To Chronic Kidney Disease 2472 100X 1000134Document3 pagesA Case of Idiopathic Intracranial Hypertension Due To Chronic Kidney Disease 2472 100X 1000134Betül SulubulutNo ratings yet
- Ischemic Stroke in A Young Male Patient in Rural Uganda, Case Report.Document3 pagesIschemic Stroke in A Young Male Patient in Rural Uganda, Case Report.International Journal of Innovative Science and Research TechnologyNo ratings yet
- Acute Ischemic Stroke: Clinical PracticeDocument8 pagesAcute Ischemic Stroke: Clinical PracticeDorina FrunzeNo ratings yet
- Edlow 2018Document15 pagesEdlow 2018arianaNo ratings yet
- Lupus Myocarditis Presenting As Acute Congestive Heart FailureDocument3 pagesLupus Myocarditis Presenting As Acute Congestive Heart FailurehafifanisaNo ratings yet
- 2020 Can Ocular OCT Findings Be As A Predictor For End-Organ Damage in Systemic Hypertension?Document5 pages2020 Can Ocular OCT Findings Be As A Predictor For End-Organ Damage in Systemic Hypertension?StefanieNo ratings yet
- Effects of An Antithrombin Drug in Patients With Subacute Exacerbations of Binswanger DiseaseDocument4 pagesEffects of An Antithrombin Drug in Patients With Subacute Exacerbations of Binswanger DiseaseFortune FireNo ratings yet
- Comorbidities in Patients With Gout in R f99b8513Document3 pagesComorbidities in Patients With Gout in R f99b8513Natasya SaraswatiNo ratings yet
- Sydenham Chorea 2019Document3 pagesSydenham Chorea 2019Hajrin PajriNo ratings yet
- Clinical Approach to Sudden Cardiac Death SyndromesFrom EverandClinical Approach to Sudden Cardiac Death SyndromesRamon BrugadaNo ratings yet
- Postural Tachycardia Syndrome: A Concise and Practical Guide to Management and Associated ConditionsFrom EverandPostural Tachycardia Syndrome: A Concise and Practical Guide to Management and Associated ConditionsNicholas GallNo ratings yet
- Case Study 1: Rhinitis AllergyDocument18 pagesCase Study 1: Rhinitis AllergyAsfiksia NeonatorumNo ratings yet
- Assessment Nursing Diagnosis Planning Intervention Rationale EvaluationDocument6 pagesAssessment Nursing Diagnosis Planning Intervention Rationale EvaluationRenea Joy ArruejoNo ratings yet
- Veterinary Pharmacology 2011Document35 pagesVeterinary Pharmacology 2011Satnam singhNo ratings yet
- Trauma: H. Scott Bjerke, Felix Lui, Areti Tillou, and Frederick A. LuchetteDocument38 pagesTrauma: H. Scott Bjerke, Felix Lui, Areti Tillou, and Frederick A. LuchettecharoiteNo ratings yet
- Modul 4 Blok 6 Kelompok 8 - Kelainan AutoimunDocument94 pagesModul 4 Blok 6 Kelompok 8 - Kelainan AutoimunJoni ChayadiNo ratings yet
- Human Papillomavirus InfectionDocument78 pagesHuman Papillomavirus InfectionJoaquín PeñaNo ratings yet
- (BHU) DoneDocument6 pages(BHU) Donedr.venkat r singhNo ratings yet
- 2121-Article Text-9456-1-10-20230131Document8 pages2121-Article Text-9456-1-10-20230131Çağdaş BaytarNo ratings yet
- Thesis Synopsis - Naveen ReddyDocument16 pagesThesis Synopsis - Naveen ReddyNaveen ReddyNo ratings yet
- DdaDocument7 pagesDdahasyimahNo ratings yet
- Literature Review Ectopic PregnancyDocument4 pagesLiterature Review Ectopic Pregnancyakjnbowgf100% (1)
- MLT MCQDocument6 pagesMLT MCQzubair farooq50% (2)
- Ebook John Murtaghs General Practice Companion Handbook PDF Full Chapter PDFDocument67 pagesEbook John Murtaghs General Practice Companion Handbook PDF Full Chapter PDFroberto.duncan209100% (27)
- ICU One Pager NIPPVDocument1 pageICU One Pager NIPPVNicholas HelmstetterNo ratings yet
- Restoration of The Worn Dentition. Part 2Document7 pagesRestoration of The Worn Dentition. Part 2Isharajini Prasadika Subhashni GamageNo ratings yet
- Senthami sps-29.08.2011Document280 pagesSenthami sps-29.08.2011Venkatesan VidhyaNo ratings yet
- Typhon PAST WorksheetDocument2 pagesTyphon PAST WorksheetKa Chun NgNo ratings yet
- DR - Jetty - Executive Summary of Perioperative ManagementDocument28 pagesDR - Jetty - Executive Summary of Perioperative ManagementDilaNo ratings yet
- Mehlmanmedical Hy Neuro Part IDocument18 pagesMehlmanmedical Hy Neuro Part IAzlan OmarNo ratings yet
- Grand Halad Sa Kapamilya: List of ServicesDocument2 pagesGrand Halad Sa Kapamilya: List of ServicesLeo LastimosaNo ratings yet
- Principles of Emergency MedicineDocument2 pagesPrinciples of Emergency MedicinekavithaNo ratings yet
- Contoh Soal Epilepsy and SeizureDocument4 pagesContoh Soal Epilepsy and SeizurejumasriNo ratings yet
- TURBT FinalDocument52 pagesTURBT FinalMuhammad RafiNo ratings yet
- Gupta 2021Document22 pagesGupta 2021stroma januariNo ratings yet
- ISMP Voluume 15 No 10 Oct 2017-NurseAdviseERR201710Document4 pagesISMP Voluume 15 No 10 Oct 2017-NurseAdviseERR201710uss uusNo ratings yet
- RADIOTHERAPHYDocument20 pagesRADIOTHERAPHYMuhammad Hafiz KarimNo ratings yet
- DrainsDocument19 pagesDrainspaulineNo ratings yet
- First Aid Maxilofacl SurgeryDocument94 pagesFirst Aid Maxilofacl SurgerydoctorniravNo ratings yet
- Counseling Parents of Children With Disabilities: A Review of The Literature and Implications For PracticeDocument1 pageCounseling Parents of Children With Disabilities: A Review of The Literature and Implications For PracticeDiana LoiNo ratings yet
- Hypernatremia From HarrisonDocument3 pagesHypernatremia From HarrisonNobel LaureateNo ratings yet