Professional Documents
Culture Documents
Metals
Uploaded by
Nipun JhalaniOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Metals
Uploaded by
Nipun JhalaniCopyright:
Available Formats
Metals-Revision notes
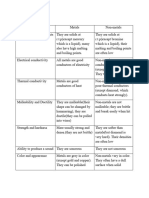
Properties of metals
Conduct heat and electricity
Are malleable (can be hammered and made into different shapes) and ductile (can be
drawn into wires)
Tend to be lustrous (shiny)
Have high density and usually have high melting points
Form positive ions through electron loss
Form basic oxides
Properties of non-metal elements
Do not conduct heat and electricity
Are brittle when solid and easily break up
Tend to be dull and nonreflective
Have low density and low melting points (many are gases at room temperature)
Form negative ions through electron gain (except for hydrogen)
Form acidic oxides
Chemical Properties of Metals
Reactivity with water
Some metals react with water, either warm or cold, or with steam
Metals that react with cold water form a metal hydroxide and hydrogen gas
metal + water → metal hydroxide + hydrogen
For example calcium:
Ca (s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
Metals that react with steam form metal oxide and hydrogen gas, for example zinc:
Zn (s) + H2O (g) → ZnO (s) + H2 (g)
Reactivity with acids
When acids and metals react, the hydrogen atom in the acid is replaced by the metal
atom to produce a salt and hydrogen gas, for example iron:
metal + acid → salt + hydrogen
Fe (s) + 2HCl (aq) → FeCl2 (aq) + H2 (g)
Reactivity with oxygen
Unreactive metals such as gold and platinum do not react with oxygen.
When metals react with oxygen a metal oxide is formed, for example copper:
metal + oxygen → metal oxide
2Cu (s) + O2 (g) → 2CuO (s)
Uses of Metals
Uses of Aluminium
Uses of Copper
Properties & Uses of Alloys
An alloy is a mixture of two or more metals or metal with a non-metal such as
carbon.
Common alloys and their uses
Brass is an alloy of copper and zinc and is much stronger than either metal
o It is used in musical instruments, ornaments and door knobs
Stainless steel is a mixture of iron and other elements, for example, chromium,
nickel and carbon
o It is used in cutlery because of its hardness and resistance to corrosion
Alloys of iron with tungsten are extremely hard and resistant to
high temperatures
Alloys of iron mixed with chromium or nickel are resistant to corrosion
Aluminium is mixed with copper, manganese and silicon for aircraft body
production as the alloy is stronger but still has a low density
You might also like
- Metals IGCSE NotesDocument27 pagesMetals IGCSE NotesMisbah Kamran100% (1)
- Metals and Non MetalsDocument57 pagesMetals and Non MetalsLOLBOINo ratings yet
- TC LG PDFDocument253 pagesTC LG PDFKieron Ivan M. Gutierrez100% (2)
- Metals and Non MetalsDocument9 pagesMetals and Non MetalsKrishna SharmaNo ratings yet
- METALS and NON-METALSDocument24 pagesMETALS and NON-METALSTushti Ramlogan100% (1)
- IUPAC - Names of Ions and RadicalsDocument16 pagesIUPAC - Names of Ions and RadicalsSD UserNo ratings yet
- Heterogeneous Reactor DesignDocument267 pagesHeterogeneous Reactor DesignS S S REDDY100% (1)
- Chemistry IB TOPIC 1 QuestionsDocument25 pagesChemistry IB TOPIC 1 QuestionsJennifer Chu100% (1)
- Set A Multiple Choice Questions Metals and Non-MetalsDocument7 pagesSet A Multiple Choice Questions Metals and Non-MetalsskandhaNo ratings yet
- CSEC Chem Metals Chemistry of Gardening EtcDocument25 pagesCSEC Chem Metals Chemistry of Gardening Etcdela2100% (2)
- Metals and Non Metals - NotesDocument13 pagesMetals and Non Metals - NotesmittalshivamNo ratings yet
- The Periodic Table of ElementsDocument41 pagesThe Periodic Table of ElementsPawan GoswamiNo ratings yet
- Metals and Non Metals - Shobhit NirwanDocument17 pagesMetals and Non Metals - Shobhit NirwanBhaskar 8287No ratings yet
- Why Do Metals Rust? An Easy Read Chemistry Book for Kids | Children's Chemistry BooksFrom EverandWhy Do Metals Rust? An Easy Read Chemistry Book for Kids | Children's Chemistry BooksNo ratings yet
- Metals and Non-MetalsDocument14 pagesMetals and Non-MetalsKunal HazarikaNo ratings yet
- Metals and Non MetalsDocument15 pagesMetals and Non Metals2erwr100% (2)
- AQA GCSE Triple C7 Test 5 Advanced QPDocument20 pagesAQA GCSE Triple C7 Test 5 Advanced QPryanNo ratings yet
- Metals and Non-MetalsDocument23 pagesMetals and Non-MetalsAnonymous ufMAGXcskMNo ratings yet
- MetalsDocument7 pagesMetalschongkee56No ratings yet
- Metals NotesDocument4 pagesMetals NotesDiyaNo ratings yet
- Metals, Non Metals, and MetallurgyDocument13 pagesMetals, Non Metals, and MetallurgyM. Amebari NongsiejNo ratings yet
- Chapter 3 - Metals and Non MetalsDocument17 pagesChapter 3 - Metals and Non Metalskush96122No ratings yet
- Metal and NonmetalDocument26 pagesMetal and NonmetalSudhanshu Sekhar PandaNo ratings yet
- Reading Material by NVS TeacherDocument12 pagesReading Material by NVS Teacher10E Yuvan Sarabeshan Thirumeninathan [3383]No ratings yet
- 3 NOV Class 10 Metals and Non-Metals ChemDocument40 pages3 NOV Class 10 Metals and Non-Metals Chemgourav kaliaNo ratings yet
- Chapter 3 Class 10Document15 pagesChapter 3 Class 10Dishu SinghNo ratings yet
- 0620 Properties of MetalsDocument10 pages0620 Properties of MetalsTadisa ChiwiraNo ratings yet
- Metals and Non-Metals - Chemical Properties NotesDocument13 pagesMetals and Non-Metals - Chemical Properties NotesDhyan ShahNo ratings yet
- Metals and The Reactiviy Series-NotesDocument4 pagesMetals and The Reactiviy Series-NotesAhmad Shafiq ZiaNo ratings yet
- 8th Metal and Non MetalDocument8 pages8th Metal and Non MetalsubrotokumarmohantaNo ratings yet
- Metals OlevleDocument9 pagesMetals OlevleaayannisarNo ratings yet
- Materials Metals and Non-MetalsDocument5 pagesMaterials Metals and Non-Metalssidhantm823No ratings yet
- Geography Lesson 2Document5 pagesGeography Lesson 2indaneNo ratings yet
- Silver Hills Public School, Kozhikode: Class NotesDocument12 pagesSilver Hills Public School, Kozhikode: Class NotesMaria Joe.kNo ratings yet
- Metal and Non Metal Class VIIIDocument9 pagesMetal and Non Metal Class VIIIamrendraNo ratings yet
- Mainly in Group I, Group II, and The Transition Block - Those The Staircase LineDocument14 pagesMainly in Group I, Group II, and The Transition Block - Those The Staircase LineOrderPlace AccountNo ratings yet
- Metal and Non Metal Class VIIIDocument9 pagesMetal and Non Metal Class VIIIDr. Amrendra JhaNo ratings yet
- 10.1 Properties of MetalsDocument11 pages10.1 Properties of Metalss103038No ratings yet
- Metals PropertiesDocument11 pagesMetals PropertiesLinta AntonyNo ratings yet
- Metals and Non Metals Class 7Document7 pagesMetals and Non Metals Class 7adinathdinesh99No ratings yet
- Metals and Non MetalsDocument9 pagesMetals and Non Metalsbhumika motiyaniNo ratings yet
- Metals and Non-Metals Class 10 Notes Science Chapter 3Document3 pagesMetals and Non-Metals Class 10 Notes Science Chapter 3samNo ratings yet
- METALDocument5 pagesMETALSachin YadavNo ratings yet
- Metals PreliminaryDocument26 pagesMetals PreliminaryIra Katriel NunagNo ratings yet
- Metal Notes #1Document7 pagesMetal Notes #1swcaptain2008No ratings yet
- Metals and Non-MetalsDocument23 pagesMetals and Non-MetalsPetrichorNo ratings yet
- Uydz Uw WV USKa N61 MM JC 4Document6 pagesUydz Uw WV USKa N61 MM JC 4varshatagade126No ratings yet
- Metals and Non MetalsDocument72 pagesMetals and Non MetalssimoneNo ratings yet
- Metals ReviewerDocument8 pagesMetals ReviewerCyber DomingoNo ratings yet
- Padhle 10th - Metal & Non-Metals Lecture SlidesDocument25 pagesPadhle 10th - Metal & Non-Metals Lecture SlidesBitan DasNo ratings yet
- Chapter 3 Metals and NonmetalsDocument37 pagesChapter 3 Metals and NonmetalsVibi VibesNo ratings yet
- 3 NOV Class 10 Metals and Non-Metals ChemDocument40 pages3 NOV Class 10 Metals and Non-Metals Chemgourav kaliaNo ratings yet
- 1 Metals and NonmetalsDocument13 pages1 Metals and Nonmetalsthinkiit100% (1)
- Chapter 4Document16 pagesChapter 4Bhavya JangidNo ratings yet
- Lec 05 - Chemistry - Metals and Non-MetalsDocument4 pagesLec 05 - Chemistry - Metals and Non-MetalsManjyot KourNo ratings yet
- Metals and Their Properties - Physical and ChemicalDocument5 pagesMetals and Their Properties - Physical and Chemicalcourtz911No ratings yet
- Metals: Properties of Metals Extraction of Metals Uses of MetalsDocument29 pagesMetals: Properties of Metals Extraction of Metals Uses of MetalsdhawandNo ratings yet
- Metals and Non MetalsDocument9 pagesMetals and Non Metalsvishwath donepudiNo ratings yet
- Class 10 Metals and Non Metals NotesDocument12 pagesClass 10 Metals and Non Metals NotesShreyash VishwakarmaNo ratings yet
- Chapter 13, 14 - Metals PDFDocument9 pagesChapter 13, 14 - Metals PDFAarush SharmaNo ratings yet
- Notes On Materials Metals and Non MetalsDocument6 pagesNotes On Materials Metals and Non Metalsmatho logyNo ratings yet
- AssignmentDocument13 pagesAssignmentfarusadiq.77No ratings yet
- Metals and Non-MetalsDocument9 pagesMetals and Non-Metalsmonkey.luffy.kenNo ratings yet
- Class10 Science Notes Chapte3Document9 pagesClass10 Science Notes Chapte3PallaviGupta100% (1)
- Ref: Corrosion: AlloysDocument7 pagesRef: Corrosion: AlloysAhmed shakilNo ratings yet
- Properties of MetalsDocument4 pagesProperties of MetalsjahangirNo ratings yet
- Article For EnglishDocument1 pageArticle For EnglishNipun JhalaniNo ratings yet
- Speech by Narendra Modi in UN General Assembly-FullDocument5 pagesSpeech by Narendra Modi in UN General Assembly-FullNipun JhalaniNo ratings yet
- Cambridge IGCSE™: French 0520/42 March 2020Document42 pagesCambridge IGCSE™: French 0520/42 March 2020Nipun JhalaniNo ratings yet
- Cambridge IGCSE™: French 0520/22 March 2020Document13 pagesCambridge IGCSE™: French 0520/22 March 2020Nipun JhalaniNo ratings yet
- Cambridge IGCSE™: French 0520/12 March 2020Document14 pagesCambridge IGCSE™: French 0520/12 March 2020Nipun JhalaniNo ratings yet
- Cambridge IGCSE: FRENCH 0520/03Document24 pagesCambridge IGCSE: FRENCH 0520/03Nipun JhalaniNo ratings yet
- French: Paper 0520/12 ListeningDocument14 pagesFrench: Paper 0520/12 ListeningNipun JhalaniNo ratings yet
- Grade Thresholds - March 2020: Cambridge IGCSE French (Foreign Language) (0520)Document1 pageGrade Thresholds - March 2020: Cambridge IGCSE French (Foreign Language) (0520)Nipun JhalaniNo ratings yet
- HDD and SSD - Comparative Study For IGCSEDocument7 pagesHDD and SSD - Comparative Study For IGCSENipun JhalaniNo ratings yet
- BST Paper 1 CombinedDocument95 pagesBST Paper 1 CombinedNipun JhalaniNo ratings yet
- Chemistry Notes Class 10Document8 pagesChemistry Notes Class 10nejihyuga997No ratings yet
- IGCSE Chemistry CalculationsDocument27 pagesIGCSE Chemistry CalculationsMartin MulengaNo ratings yet
- Iron Low Level Total - AP-23 - 900Document3 pagesIron Low Level Total - AP-23 - 900wulalan wulanNo ratings yet
- Wa0017.Document7 pagesWa0017.Nischal Reddy SareddyNo ratings yet
- 5070 s11 QP 12Document16 pages5070 s11 QP 12mstudy123456No ratings yet
- GenChem11 Q1 M2-1 071654Document33 pagesGenChem11 Q1 M2-1 071654edjannaciongayo2314No ratings yet
- 1chemical Reactions & Equations Top 25 Questions Prashant KiradDocument12 pages1chemical Reactions & Equations Top 25 Questions Prashant KiradKshitiz sharma100% (1)
- Year 10 EOT3 Revison Booklet With AnswerDocument99 pagesYear 10 EOT3 Revison Booklet With AnswerAdamNo ratings yet
- Effects of Boron Doping in Low - and High-surface-Area Carbon PowdersDocument12 pagesEffects of Boron Doping in Low - and High-surface-Area Carbon Powdersvictor romeroNo ratings yet
- Vamac Formulating and Compounding OverviewDocument6 pagesVamac Formulating and Compounding Overviewchethugowda7No ratings yet
- Chemistry: Written Examination 2Document17 pagesChemistry: Written Examination 2luctonNo ratings yet
- Corrosion of Archaeological Artefact Made of Forged IronDocument8 pagesCorrosion of Archaeological Artefact Made of Forged IronsoloipseNo ratings yet
- Revision Year 9 ChemistryDocument6 pagesRevision Year 9 ChemistryNesrine HaifNo ratings yet
- Parker O-Ring Size ChartDocument25 pagesParker O-Ring Size ChartChinh PhamNo ratings yet
- Read These Instructions: Topic Mock-EDocument24 pagesRead These Instructions: Topic Mock-EFaiyad Ahmed MasnoonNo ratings yet
- Cambridge International AS & A Level: Chemistry 9701/11Document16 pagesCambridge International AS & A Level: Chemistry 9701/11윤소리No ratings yet
- University of Cambridge International Examinations General Certificate of Education Ordinary LevelDocument16 pagesUniversity of Cambridge International Examinations General Certificate of Education Ordinary Levelmstudy123456No ratings yet
- 0439 s15 QP 33Document12 pages0439 s15 QP 33Hamza JavedNo ratings yet
- MYP 3 Criterion B-RustingDocument5 pagesMYP 3 Criterion B-Rustingwama ojha0% (1)
- Uganda Lower Secondary Certificate of Education. Chemistry Senior Two End of Term One 2023Document12 pagesUganda Lower Secondary Certificate of Education. Chemistry Senior Two End of Term One 2023Owani Jimmy100% (1)
- 0620 s11 QP 31Document12 pages0620 s11 QP 31Kelso ZwariyaNo ratings yet
- 11.2 - Ingliskeelne NimekiriDocument37 pages11.2 - Ingliskeelne NimekiriMichael MacDonaldNo ratings yet
- Chemical Reactions and Equations Activity Based Question 10thDocument18 pagesChemical Reactions and Equations Activity Based Question 10thBharatharaj123No ratings yet