Professional Documents
Culture Documents
Senyawa Aromatik 2
Uploaded by
lenrokmartdwi23Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Senyawa Aromatik 2
Uploaded by
lenrokmartdwi23Copyright:
Available Formats
340 Chapter 11 Unsaturated Hydrocarbons
Recycled HDPE is converted to Tyvek, an insulating wrap used in new housing construction, and
recycled PET is used to make fibers for fleece clothing and carpeting. Currently about 23% of all
plastics are recycled in the United States.
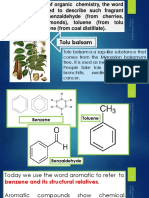
11.8 Aromatic Compounds
Aromatic compounds represent another example of unsaturated hydrocarbons. Aromatic com-
pounds were originally named because many simple compounds in this family have characteris-
tic odors. Today, the word aromatic refers to compounds that contain a benzene ring, or rings
that react in a similar fashion to benzene.
HEALTH NOTE Benzene, the simplest and most widely known aromatic compound, contains a six-membered
ring and three double bonds. Since each carbon of the ring is also bonded to a hydrogen atom,
the molecular formula for benzene is C6H6. Each carbon is surrounded by three groups, making
it trigonal planar. Thus, benzene is a planar molecule, and all bond angles are 120°.
H
H C H
C C
= = 120°
C C
H C H
Tamoxifen, a potent anticancer drug H
sold under the trade name of Novaldex, benzene planar molecule
contains three benzene rings. C6H6
OCH2CH2N(CH3)2
Although benzene is drawn with a six-membered ring and three double bonds, there are two dif-
ferent ways to arrange the double bonds so that they alternate with single bonds around the ring.
Each of these representations is equivalent.
C C tamoxifen
CH3CH2 or
This bond is a single bond in one structure
and a double bond in the second structure.
The physical properties of aromatic hydrocarbons are similar to other hydrocarbons—they have
low melting points and boiling points and are water insoluble.
11.9 Nomenclature of Benzene Derivatives
Many organic molecules contain a benzene ring with one or more substituents, so we must learn
how to name them.
11.9A Monosubstituted Benzenes
To name a benzene ring with one substituent, name the substituent and add the word benzene.
Carbon substituents are named as alkyl groups. When a halogen is a substituent, name the
halogen by changing the -ine ending of the name of the halogen to the suffix -o; for example,
chlorine → chloro.
CH2CH3 CH2CH2CH2CH3 Cl
ethyl group butyl group chloro group
ethylbenzene butylbenzene chlorobenzene
smi11153_ch11_322-352.indd 340 10/7/10 1:53 PM
11.9 Nomenclature of Benzene Derivatives 341
Many monosubstituted benzenes, such as those with methyl (CH3–), hydroxyl (–OH), and amino
(–NH2) groups, have common names that you must learn, too.
CH3 OH NH2
toluene phenol aniline
(methylbenzene) (hydroxybenzene) (aminobenzene)
11.9B Disubstituted Benzenes
There are three different ways that two groups can be attached to a benzene ring, so a prefix—
ortho, meta, or para—is used to designate the relative position of the two substituents. Ortho,
meta, and para are generally abbreviated as o, m, and p, respectively.
1,2-Disubstituted benzene 1,3-Disubstituted benzene 1,4-Disubstituted benzene
ortho isomer meta isomer para isomer
CH2CH3 CH2CH3 CH2CH3
CH2CH3
CH2CH3
CH2CH3
o-diethylbenzene m-diethylbenzene p-diethylbenzene
or or or
1,2-diethylbenzene 1,3-diethylbenzene 1,4-diethylbenzene
If the two groups on the benzene ring are different, alphabetize the name of the substituents
The pain reliever acetaminophen preceding the word benzene. If one of the substituents is part of a common root, name the mol-
(trade name Tylenol) contains a para- ecule as a derivative of that monosubstituted benzene.
disubstituted benzene ring.
H Alphabetize two different substituent names: Use a common root name:
N CH3 Br CH2CH3 toluene phenol
C Cl
Cl
O
HO
Br CH3 OH
acetaminophen
F
(Trade name: Tylenol)
o-bromochloro- m-ethylfluoro-
benzene benzene p-bromotoluene o-chlorophenol
11.9C Polysubstituted Benzenes
For three or more substituents on a benzene ring:
1. Number to give the lowest possible numbers around the ring.
2. Alphabetize the substituent names.
3. When substituents are part of common roots, name the molecule as a derivative of that
monosubstituted benzene. The substituent that comprises the common root is located at C1,
but the “1” is omitted from the name.
smi11153_ch11_322-352.indd 341 10/7/10 1:53 PM
342 Chapter 11 Unsaturated Hydrocarbons
Examples of naming polysubstituted benzenes
1
NH2
CH2CH3 Cl 1
2
Cl CH2CH2CH3 Cl
4 2 5
• Name the molecule as a derivative of the
• Assign the lowest set of numbers. common root aniline.
• Alphabetize the names of all the • Designate the position of the NH2 group as “1,”
substituents. and then assign the lowest possible set of
numbers to the other substituents.
4-chloro-1-ethyl-2-propylbenzene 2,5-dichloroaniline
SAMPLE PROBLEM 11.7
Name each of the following aromatic compounds.
CH3CH2 CH2CH2CH3 CH3 Cl
a. b.
Br
Analysis
Name the substituents on the benzene ring. With two groups, alphabetize the substituent names and
use the prefix ortho, meta, or para to indicate their location. With three substituents, alphabetize the
substituent names, and number to give the lowest set of numbers.
Solution
a. CH3CH2 CH2CH2CH3 • The two substituents are located 1,3- or meta to
each other.
ethyl propyl
group group • Alphabetize the e of ethyl before the p of propyl.
Answer: m-ethylpropylbenzene
1
b. CH3 Cl • Since a CH3– group is bonded to the ring, name the
3
molecule as a derivative of toluene.
4
Br • Place the CH3 group at the “1” position, and
number to give the lowest set of numbers.
toluene
Answer: 4-bromo-3-chlorotoluene
PROBLEM 11.18
Give the IUPAC name of each compound.
OH
CH2CH2CH3
a. c.
CH2CH2CH2CH3
CH3
CH2CH3 Br
b. d.
I Cl
PROBLEM 11.19
Draw the structure corresponding to each name.
a. pentylbenzene c. m-bromoaniline
b. o-dichlorobenzene d. 4-chloro-1,2-diethylbenzene
smi11153_ch11_322-352.indd 342 10/7/10 1:53 PM
11.10 FOCUS ON HEALTH & MEDICINE: Sunscreens and Antioxidants 343
11.10 FOCUS ON HEALTH & MEDICINE
Sunscreens and Antioxidants
HEALTH NOTE 11.10A Sunscreens
All commercially available sunscreens contain a benzene ring. A sunscreen absorbs ultraviolet
radiation and thus shields the skin for a time from its harmful effects. Two sunscreens that have
been used for this purpose are p-aminobenzoic acid (PABA) and Padimate O.
O O
H2N C (CH3)2N C
OH OCH2CHCH2CH2CH2CH3
p-aminobenzoic acid Padimate O CH2CH3
(PABA)
PROBLEM 11.20
Identify the functional groups in each sunscreen: (a) PABA; (b) Padimate O.
PROBLEM 11.21
Which of the following compounds might be an ingredient in a commercial sunscreen? Explain why
or why not.
O O
a. b.
Commercial sunscreens are given an CH3O OH
SPF rating (sun protection factor),
according to the amount of sunscreen
present. The higher the number, the 11.10B Phenols as Antioxidants
greater the protection.
A wide variety of phenols, compounds that contain a hydroxyl group bonded to a benzene ring,
occur in nature. Vanillin from the vanilla bean is a phenol, as is curcumin, a yellow pigment
isolated from turmeric, a tropical perennial in the ginger family and a principal ingredient in
curry powder. Curcumin has long been used as an anti-inflammatory agent in traditional eastern
medicine. In some preliminary research carried out with mice, curcumin was shown to correct the
defect that causes cystic fibrosis, a fatal genetic disease that afflicts 30,000 children and young
adults in the United States.
vanilla bean turmeric
OH H
H O O H
OCH3
C C C C
C C C
H H H
HO OH
CHO
OCH3 OCH3
vanillin curcumin
smi11153_ch11_322-352.indd 343 10/7/10 1:53 PM
344 Chapter 11 Unsaturated Hydrocarbons
HEALTH NOTE Many phenols are antioxidants, compounds that prevent unwanted oxidation reactions from
occurring. Two examples are naturally occurring vitamin E and synthetic BHT. The OH group
on the benzene ring is the key functional group that prevents oxidation reactions from
taking place.
CH3
OH
HO
(CH3)3C C(CH3)3
(CH2)3CH(CH2)3CH(CH2)3CH(CH3)2
CH3 O
CH3 CH3 CH3
CH3 CH3
BHT
vitamin E (butylated hydroxy toluene)
The purported health benefits of
antioxidants have made them a popular Vitamin E is a natural antioxidant found in fish oil, peanut oil, wheat germ, and leafy greens.
component in anti-aging formulations. Although the molecular details of its function remain obscure, it is thought that vitamin E pre-
vents the unwanted oxidation of unsaturated fatty acid residues in cell membranes. In this way,
vitamin E helps retard the aging process.
HEALTH NOTE
Synthetic antioxidants such as BHT—butylated hydroxy toluene—are added to packaged and
prepared foods to prevent oxidation and spoilage. BHT is a common additive in breakfast cereals.
PROBLEM 11.22
Which of the following compounds might be antioxidants?
a. CH3 CH(CH3)2 b. curcumin c. CH3 CH3
OH p-xylene
Nuts are an excellent source of vitamin
(gasoline additive)
E. menthol
KEY TERMS
Addition reaction (11.5) Fatty acid (11.3) Para isomer (11.9)
Alkene (11.1) Hydration (11.5) Partial hydrogenation (11.6)
Alkyne (11.1) Hydrogenation (11.5) Polymer (11.7)
Antioxidant (11.10) Meta isomer (11.9) Polymerization (11.7)
Aromatic compound (11.8) Monomer (11.7) Stereoisomer (11.3)
Cis isomer (11.3) Oil (11.3) Trans isomer (11.3)
Fat (11.3) Ortho isomer (11.9) Unsaturated hydrocarbon (11.1)
KEY CONCEPTS
❶ What are the characteristics of alkenes, alkynes, and • Benzene, molecular formula C6H6, is the most common
aromatic compounds? aromatic hydrocarbon. Benzene contains a six-membered ring
• Alkenes are unsaturated hydrocarbons that contain a carbon– with three double bonds, and each carbon is trigonal planar.
carbon double bond and have molecular formula CnH2n. Each (11.8)
carbon of the double bond is trigonal planar. (11.1) ❷ How are alkenes, alkynes, and substituted benzenes named?
• Alkynes are unsaturated hydrocarbons that contain a carbon– • An alkene is identified by the suffix -ene, and the carbon chain
carbon triple bond and have molecular formula CnH2n – 2. Each is numbered to give the C C the lower number. (11.2)
carbon of the triple bond is linear. (11.1)
smi11153_ch11_322-352.indd 344 10/7/10 1:53 PM
You might also like
- 6Document4 pages6Shyam TannaNo ratings yet
- Class Notes 24-Jan-2021Document7 pagesClass Notes 24-Jan-2021JJ PrakashNo ratings yet
- Chapter 4-Aromatic CompoundsDocument69 pagesChapter 4-Aromatic CompoundsNURUL BALQIS DZULKIFLINo ratings yet
- Benzene & Its Derivatives: (The Class of Compounds, Which Contains Benzene or Substituted Derivative of Benzene)Document17 pagesBenzene & Its Derivatives: (The Class of Compounds, Which Contains Benzene or Substituted Derivative of Benzene)justin youngNo ratings yet
- KimiDocument29 pagesKimiDejan KrajaNo ratings yet
- Aromatic Hydrocarbons Pharmaceutical Organic Chemistry Porg111Document9 pagesAromatic Hydrocarbons Pharmaceutical Organic Chemistry Porg111AnnaGueseNo ratings yet
- BenzeneDocument14 pagesBenzeneJueeli More100% (1)
- Reactividad Del BencenoDocument3 pagesReactividad Del BencenoRoberto GoncalvesNo ratings yet
- LEC 19 Nomen-Culture and Structure of BenzeneDocument16 pagesLEC 19 Nomen-Culture and Structure of BenzeneIftikhar Ud DinNo ratings yet
- Chapter 5 AromaticDocument76 pagesChapter 5 AromaticMELVINDO JACOBNo ratings yet
- Chemistry of Aromatics CompoundsDocument16 pagesChemistry of Aromatics CompoundsOfudje Edwin AndrewNo ratings yet
- Ch2 Hydrocarbon AromaticDocument36 pagesCh2 Hydrocarbon AromaticAlimah Azeli100% (2)
- Lesson 4 Aromatic HCDocument24 pagesLesson 4 Aromatic HCEricka LaluzNo ratings yet
- SCH 4U The Structure of BenzeneDocument21 pagesSCH 4U The Structure of Benzenediego.lopez1870No ratings yet
- BenzeneDocument21 pagesBenzeneosamakhan8967No ratings yet
- Benzene and Aromaticity (2nd Year Ndaweni)Document54 pagesBenzene and Aromaticity (2nd Year Ndaweni)Mbali MazongweNo ratings yet
- Mod 4 Revision Guide 6 BenzeneDocument4 pagesMod 4 Revision Guide 6 BenzenenomoszengNo ratings yet
- Lec-4 Aromatic HydrocarbonsDocument17 pagesLec-4 Aromatic HydrocarbonsTalal Ahmed Awad MohammedNo ratings yet
- 3 Organic Chemistry - BenzeneDocument39 pages3 Organic Chemistry - BenzeneIsuriy AdasuriyaNo ratings yet
- Nomenclature: ArenesDocument10 pagesNomenclature: Arenesangi gongopolNo ratings yet
- Organic Chemistry Lecture 2 2022 Modif.Document60 pagesOrganic Chemistry Lecture 2 2022 Modif.Fady FadyNo ratings yet
- Corrected Fundamentals of Organic ChemistryDocument71 pagesCorrected Fundamentals of Organic ChemistryDAM2120No ratings yet
- Chapter 4 Aromatic HydrocarbonsDocument34 pagesChapter 4 Aromatic HydrocarbonsAbdirashid Adam IsakNo ratings yet
- Chapter 4-Aromatic CompoundsDocument48 pagesChapter 4-Aromatic CompoundsNur Ayu Nadhirah Bt YahyaNo ratings yet
- Lecture 7-8 Term 3, AY 22-23Document31 pagesLecture 7-8 Term 3, AY 22-23LujainNo ratings yet
- Dienes & Aromatic Compounds, FNDocument60 pagesDienes & Aromatic Compounds, FNMuzahidul IslamNo ratings yet
- Chapter 4 Aromatic CompoundsDocument61 pagesChapter 4 Aromatic CompoundsapayNo ratings yet
- St. Andrew's Junior College H2 Chemistry 2013 Organic Chemistry Lecture Notes 5 ArenesDocument26 pagesSt. Andrew's Junior College H2 Chemistry 2013 Organic Chemistry Lecture Notes 5 ArenesArvin LiangdyNo ratings yet
- AaasdDocument5 pagesAaasdmaeallysa07No ratings yet
- 4 Benzene PDFDocument90 pages4 Benzene PDFCrishen VinzonNo ratings yet
- WEEK 10 Aromatic HydrocarbonDocument26 pagesWEEK 10 Aromatic HydrocarbonChris Angelo De GuzmanNo ratings yet
- W4 BENZENE Nomenclature Stability Physical & Chemical Properties PDFDocument22 pagesW4 BENZENE Nomenclature Stability Physical & Chemical Properties PDFathyrahNo ratings yet
- Chapter 4 Aromatic CompoundsDocument55 pagesChapter 4 Aromatic CompoundsKonoli NuingNo ratings yet
- 6.0 Benzene 2020-2021Document69 pages6.0 Benzene 2020-2021Suhaila Arzimi100% (1)
- Benzene Aromatic CompoundsDocument14 pagesBenzene Aromatic CompoundsBook of Life fgfhfghfghfghNo ratings yet
- Organic Chemistry Module 4Document5 pagesOrganic Chemistry Module 4Josephine TeroNo ratings yet
- 2007 Chap 13 NotesDocument6 pages2007 Chap 13 NotesSonu TadaiyaNo ratings yet
- Benzene and Its Derivatives: Key QuestionsDocument58 pagesBenzene and Its Derivatives: Key QuestionsBrian GichanaNo ratings yet
- Aromatic Hydrocarbons Unit For SuccessDocument54 pagesAromatic Hydrocarbons Unit For SuccessN210084 CHOULA MANIKANTANo ratings yet
- OCR Chemistry NotesDocument10 pagesOCR Chemistry NotesJack WoodNo ratings yet
- Chapter 4Document28 pagesChapter 4c4.arsyadNo ratings yet
- Reaction of Alkenes and Alkynes For StudentsDocument53 pagesReaction of Alkenes and Alkynes For StudentsGlen MangaliNo ratings yet
- Aromatic CompoundsDocument25 pagesAromatic CompoundsElizabeth Vivar100% (1)
- Organic Chemistry,: Benzene & AromaticsDocument41 pagesOrganic Chemistry,: Benzene & AromaticsRIZKI ALDINO AHMAD 1506723912No ratings yet
- CHY2023 - Unit 3 Aromatic HydrocarbonsDocument75 pagesCHY2023 - Unit 3 Aromatic HydrocarbonsZhori Duberry100% (1)
- Chem263 Oct5 Notes 2010Document10 pagesChem263 Oct5 Notes 2010awais gujjarNo ratings yet
- Benzene Part 1 CPiDocument12 pagesBenzene Part 1 CPiShofwa AnnisaNo ratings yet
- Chapter 17 - BENZENE AND AROMATICSDocument28 pagesChapter 17 - BENZENE AND AROMATICSsalman alfarizziNo ratings yet
- Benzene MilanaDocument61 pagesBenzene MilanaMilana WalujoNo ratings yet
- Benzene and Its DerivativesDocument21 pagesBenzene and Its DerivativesChristland De jesusNo ratings yet
- NEPHAR 109 Chapter14Document24 pagesNEPHAR 109 Chapter14Amirabbas SaffariNo ratings yet
- Aromatic Compounds: NamingDocument13 pagesAromatic Compounds: NamingTanzimNo ratings yet
- Benzene and Its Derivatives 1Document22 pagesBenzene and Its Derivatives 1Arielle Ciara BajadaNo ratings yet
- Organic Chemistry For USTH Students Benzene and Aromatic SystemsDocument69 pagesOrganic Chemistry For USTH Students Benzene and Aromatic SystemsHoàng Hiệp100% (1)
- Bab 10a Benzene and AromaticityDocument41 pagesBab 10a Benzene and AromaticityAl KahfiNo ratings yet
- Aromatic CompoundsDocument27 pagesAromatic CompoundsScience AcademyNo ratings yet
- Aromatic Compounds 2Document28 pagesAromatic Compounds 2Umar TahirNo ratings yet
- Benzene and It'S Derivatives: It Has 6 Carbons With 3 Double BondsDocument11 pagesBenzene and It'S Derivatives: It Has 6 Carbons With 3 Double BondsJohn RavenNo ratings yet
- Concerning Amines: Their Properties, Preparation and ReactionsFrom EverandConcerning Amines: Their Properties, Preparation and ReactionsRating: 2.5 out of 5 stars2.5/5 (2)
- Reaction Quotient: Trial 1 Trial 2Document14 pagesReaction Quotient: Trial 1 Trial 2criselda macabuhayNo ratings yet
- The Fine OF: Structure Human CementumDocument44 pagesThe Fine OF: Structure Human CementumMarlon Cespedes AlccaNo ratings yet
- Fluidized Bed Reactor DesignDocument22 pagesFluidized Bed Reactor Designs barmanNo ratings yet
- Af 163-2Document10 pagesAf 163-2lacsmm982No ratings yet
- HELMER DH8 User ManualDocument30 pagesHELMER DH8 User ManualSamson AyalewNo ratings yet
- ADIP RefineryDocument2 pagesADIP RefineryVenkatesh Kumar RamanujamNo ratings yet
- Lecture Planner - ChemistryDocument7 pagesLecture Planner - ChemistryRajat BhartiNo ratings yet
- Predicting Radiative Heat Uxes and Ammability Envelopes From Unintended Releases of HydrogenDocument16 pagesPredicting Radiative Heat Uxes and Ammability Envelopes From Unintended Releases of HydrogenNafees VakilNo ratings yet
- Minimum Reflux Ratio Calculation by Underwood Method: Solutions For R&D To DesignDocument8 pagesMinimum Reflux Ratio Calculation by Underwood Method: Solutions For R&D To Designzhexuanliuoutlook.comNo ratings yet
- LipidsDocument44 pagesLipidsMilena De CresentNo ratings yet
- Foam EOR As An Optimization Technique For Gas EOR - A Comprehensive Review of Laboratory and Field ImplementationsDocument52 pagesFoam EOR As An Optimization Technique For Gas EOR - A Comprehensive Review of Laboratory and Field ImplementationsAshrafNo ratings yet
- Lab AssignmentDocument8 pagesLab AssignmentRaihanNo ratings yet
- Processes: Performance Comparison of Industrially Produced Formaldehyde Using Two DiDocument12 pagesProcesses: Performance Comparison of Industrially Produced Formaldehyde Using Two DiMohammed FaiqNo ratings yet
- Sika Monotop 412Document4 pagesSika Monotop 412Chee Soon LeeNo ratings yet
- Fdocuments - in - The Art of Violin Making 1999 253 Pages Chris Johnson Art of Violin MakingDocument21 pagesFdocuments - in - The Art of Violin Making 1999 253 Pages Chris Johnson Art of Violin MakingAurelio AurelinoNo ratings yet
- PRE-MID TERM EXAM (2022 - 23) Subject: Science Class: X Time: 1Hr30Min M.M - 40Document6 pagesPRE-MID TERM EXAM (2022 - 23) Subject: Science Class: X Time: 1Hr30Min M.M - 40VatsalyaNo ratings yet
- CH 7 Statistical Data Treatment and EvaluationDocument30 pagesCH 7 Statistical Data Treatment and Evaluationjhzlbarrera09No ratings yet
- PB Class X Science 2023-24Document9 pagesPB Class X Science 2023-24smarty boysNo ratings yet
- Iupac Nomenclature Rules 1Document1 pageIupac Nomenclature Rules 1CjES EvaristoNo ratings yet
- Mutifa 01.10.22Document3 pagesMutifa 01.10.22Apotek Mirza Praktek Dokter UmumNo ratings yet
- Ipl Part Test - 2 Final Syllabus (09.01.2024 0 10.01.2024)Document1 pageIpl Part Test - 2 Final Syllabus (09.01.2024 0 10.01.2024)murali.trichyNo ratings yet
- Archive of SIDDocument9 pagesArchive of SIDFarhan RamadhanNo ratings yet
- PressureTest AquasignKISS 123 PDFDocument6 pagesPressureTest AquasignKISS 123 PDFBruno César LevitaNo ratings yet
- Thermostatic Control ValveDocument10 pagesThermostatic Control ValveAbu Yussif AlaboodiNo ratings yet
- Practical Techniques Booklet Qm1hekDocument49 pagesPractical Techniques Booklet Qm1hekzena Al-SarawanNo ratings yet
- 11 - Group 2Document37 pages11 - Group 2enderothNo ratings yet
- BV Sheet Ile Inspection ReportDocument7 pagesBV Sheet Ile Inspection ReportLego EdrisaNo ratings yet
- Ichem Lab Post LabDocument3 pagesIchem Lab Post LabSam Denielle TugaoenNo ratings yet
- Edited PPT On Carboxylic AcidDocument51 pagesEdited PPT On Carboxylic AcidRenante DavisNo ratings yet
- Aits 2223 PT III Jeem TD SolDocument18 pagesAits 2223 PT III Jeem TD SolNeeti VarshneyNo ratings yet