0% found this document useful (0 votes)
628 views44 pagesTopic 10 Paper 2 Part 2
This document contains a multi-part chemistry question about organic reactions and compounds. Part 1 involves questions about ethyne (C2H2) and its combustion reaction and Lewis structure. Later parts involve questions about propan-2-ol, its oxidation, and classification. Hess's law is used to calculate enthalpy changes. Polyethene formation from ethene and the conversion of ethyne to benzene are also discussed, along with standard enthalpy changes. Evidence for benzene's non-Kekulé structure is outlined.
Uploaded by
RawanMazen SharifCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
628 views44 pagesTopic 10 Paper 2 Part 2
This document contains a multi-part chemistry question about organic reactions and compounds. Part 1 involves questions about ethyne (C2H2) and its combustion reaction and Lewis structure. Later parts involve questions about propan-2-ol, its oxidation, and classification. Hess's law is used to calculate enthalpy changes. Polyethene formation from ethene and the conversion of ethyne to benzene are also discussed, along with standard enthalpy changes. Evidence for benzene's non-Kekulé structure is outlined.
Uploaded by
RawanMazen SharifCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
- Exam Questions: Contains questions related to chemistry topics requiring students to write equations and provide detailed explanations.
- Markscheme Explanations: Provides detailed marking schemes with correct answers and explanations for the exam questions.
![Topic 10 Paper 2 Part 2 [98 marks]
1a. [1 mark]
Ethyne, C2H2, reacts with oxygen in welding torches.
Write an equation for th](https://screenshots.scribd.com/Scribd/252_100_85/326/691933374/1.jpeg)
![Markscheme
«ethyne» shorter AND a greater number of shared/bonding electrons
OR
«ethyne» shorter AND stronger bond [ ]
✔
1d.](https://screenshots.scribd.com/Scribd/252_100_85/326/691933374/2.jpeg)


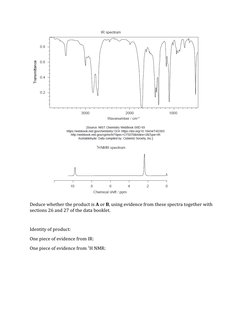

![Conditions:
Markscheme
Reagents:
acidified/H+ AND «potassium» dichromate«(VI)»/K2Cr2O7/Cr2O7
2– [ ]
✔
Conditions:
distil «th](https://screenshots.scribd.com/Scribd/252_100_85/326/691933374/7.jpeg)
![Markscheme
Any three of:
has an oxygen/O atom with a lone pair [ ]
✔
that can form hydrogen bonds/H-bonds «with water molecu](https://screenshots.scribd.com/Scribd/252_100_85/326/691933374/8.jpeg)
![2b. [2 marks]
Calculate the number of hydrogen atoms in 1.00 g of propan-2-ol.
Markscheme
«
1.00 g
(12.01×3+1.01×8+16.00) gmo](https://screenshots.scribd.com/Scribd/252_100_85/326/691933374/9.jpeg)
![Accept “secondary AND OH is attached to the second carbon in the chain”.
2d. [1 mark]
State a suitable oxidizing agent for](https://screenshots.scribd.com/Scribd/252_100_85/326/691933374/10.jpeg)
























































































