Professional Documents
Culture Documents
WhatAreTheClinicalTrialPhases Infographic 1 16NOV2021
Uploaded by
Sahil KumarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
WhatAreTheClinicalTrialPhases Infographic 1 16NOV2021
Uploaded by
Sahil KumarCopyright:
Available Formats
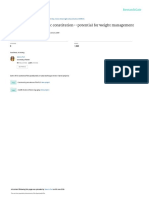
WHAT ARE THE CLINICAL TRIAL PHASES?
SAFETY EFFICACY
Is the investigational Is the investigational medication/treatment
medication/treatment safe? effective in treating the targeted condition?
• Are there side effects? • Does it relieve, reverse or stop the
• How does it affect or move through the body? progression of the condition?
• Is it safe to use at the same time as other medications? • How safe is it?
• What is the most effective dosage?
Who’s in it?
PH
Small group of healthy SE Who’s in it?
A
A
people—generally less
PH
SE
Generally 100-300 people with the
2
than 100 exact condition being studied
FOLLOW UP CONFIRMATION
PH
E
AS
AS
PH
3
E
After the investigational medication/ How does the investigational medication/
treatment is approved, how does it work
for other patients with the condition?
4 treatment compare to the standard treatment
for the condition?
• More safety/efficacy information is gathered • More effective, less effective, or the same?
• Are there long-term benefits? • Longer-term adverse effects?
• Are there long-term risks? • How does it affect quality of life, or survival?
• How might it be used along with existing treatments?
Who’s in it?
Often several thousand people Who’s in it?
who have been prescribed the Often 300-3,000 people with the
investigational medication exact condition being studied
Learn about All Trials Available Visit AbbvieClinicalTrials.com | Visit ClinicalTrials.gov | Talk to your healthcare provider Copyright © 2020 AbbVie Inc.
You might also like
- Journal Reading: Stase Ilmu THT RS Umum Daerah Sayang Cianjur Universitas Muhammadiyah Jakarta 2019Document15 pagesJournal Reading: Stase Ilmu THT RS Umum Daerah Sayang Cianjur Universitas Muhammadiyah Jakarta 2019dwi purwantiNo ratings yet
- What Your Doctor Didn't Tell You: How Complementary and Alternative Medicine Can Help Your PainFrom EverandWhat Your Doctor Didn't Tell You: How Complementary and Alternative Medicine Can Help Your PainNo ratings yet
- Entroso Shane Mtlbe Activity#1Document5 pagesEntroso Shane Mtlbe Activity#1Shane EntrosoNo ratings yet
- Gordon's Functional Health Patterns Model. The: Assessment Involves Collecting Data That Are UsedDocument5 pagesGordon's Functional Health Patterns Model. The: Assessment Involves Collecting Data That Are UsedCHINGCHONG SLAYER100% (1)
- True Wellness for Your Mind: How to Combine the Best of Western and Eastern Medicine for Optimal Health For Sleep Disorders, Anxiety, DepressionFrom EverandTrue Wellness for Your Mind: How to Combine the Best of Western and Eastern Medicine for Optimal Health For Sleep Disorders, Anxiety, DepressionNo ratings yet
- Perception of The Purok-4, Baobaoan, Butuan City Residents On The Practice of Traditional Medicine Compared To Modern MedicineDocument13 pagesPerception of The Purok-4, Baobaoan, Butuan City Residents On The Practice of Traditional Medicine Compared To Modern MedicineArnel H. CatalanNo ratings yet
- Module 20 - Complementary and Alternative MedicineDocument90 pagesModule 20 - Complementary and Alternative Medicinegeekay79No ratings yet
- Pharmacology NotesDocument2 pagesPharmacology Notessakuragi jakeNo ratings yet
- A Holistic Approach To Voice TherapyDocument8 pagesA Holistic Approach To Voice Therapybz4dzwpzw8No ratings yet
- 43Document64 pages43Pushpinder Sharma100% (1)
- CAM Self-Care Fall 2014 9.2.14Document51 pagesCAM Self-Care Fall 2014 9.2.14Leonardo RanderNo ratings yet
- Psychopharmacology: By: Urwah AliDocument17 pagesPsychopharmacology: By: Urwah AliRuqia ZafeerNo ratings yet
- ROJoson PEP Talk: Early Diagnosis and Early TreatmentDocument54 pagesROJoson PEP Talk: Early Diagnosis and Early TreatmentrojosonNo ratings yet
- Therapy For The Severe Older Adolescent and Adult StuttererDocument70 pagesTherapy For The Severe Older Adolescent and Adult StuttererDemosten100% (1)
- LTC Clinical Week One HX Assessment Rubric 20101216Document1 pageLTC Clinical Week One HX Assessment Rubric 20101216kezia_lillyNo ratings yet
- AANP HomeopathicPsychiatrySummer2014 ExtraSlides PDFDocument180 pagesAANP HomeopathicPsychiatrySummer2014 ExtraSlides PDFmajikNo ratings yet
- ClinicalexemplarDocument6 pagesClinicalexemplarapi-576596429No ratings yet
- Select Your Remedy: by Rai Bahadur Bishambhar DasDocument534 pagesSelect Your Remedy: by Rai Bahadur Bishambhar Daslilian_vera_175% (4)
- Posology: Posology It Is Divided Into Two Parts or TypesDocument6 pagesPosology: Posology It Is Divided Into Two Parts or Typesranjanamd1972No ratings yet
- Physical Examination and Health Assessment 8th EditionDocument20 pagesPhysical Examination and Health Assessment 8th EditionDerek Scott Tripp100% (1)
- Diets Based On Ayurvedic Constitution - Potential For Weight ManagementDocument89 pagesDiets Based On Ayurvedic Constitution - Potential For Weight ManagementHema NarulaNo ratings yet
- Adhering To Health ReportDocument27 pagesAdhering To Health ReportLudioman MaricelNo ratings yet
- Chapter 1 ThesisDocument9 pagesChapter 1 ThesisKharlmark Escoto DizonNo ratings yet
- 31 - Factors Associated With Adherence To Drug Therapy: Annika Bardel, GP, PHD Annika - Bardel@Pubcare - Uu.SeDocument3 pages31 - Factors Associated With Adherence To Drug Therapy: Annika Bardel, GP, PHD Annika - Bardel@Pubcare - Uu.Seeis kusmitaNo ratings yet
- MedicineDocument3 pagesMedicineMAGOS MAGOSNo ratings yet
- General Survey Nursing Assessment Cheat SheetDocument3 pagesGeneral Survey Nursing Assessment Cheat SheetVERGEL, Sophia Luis V.No ratings yet
- NCLFNP - Mr. Robert McClelland CaseDocument4 pagesNCLFNP - Mr. Robert McClelland CaseAiresh Lamao50% (2)
- Critical Appraisal JRDocument3 pagesCritical Appraisal JRRizky AgustriaNo ratings yet
- Psychiatric MedicationsDocument6 pagesPsychiatric MedicationsMr. Psycho SamNo ratings yet
- Write Down The 7 Drug CategoriesDocument1 pageWrite Down The 7 Drug Categoriesarrow3966692marklloydNo ratings yet
- PosologyDocument45 pagesPosologyShilpa SubbaiahNo ratings yet
- A Placebos and The Placebo EffectDocument7 pagesA Placebos and The Placebo EffectCOCA MAINENo ratings yet
- Midpoint Immersion ReflectionDocument3 pagesMidpoint Immersion Reflectionapi-433883631No ratings yet
- Core TranscriptDocument46 pagesCore TranscriptSamNo ratings yet
- YIN QIANHE ON LONGEVITY - Brennan Translation PDFDocument182 pagesYIN QIANHE ON LONGEVITY - Brennan Translation PDFfofofofo100% (4)
- Integrative, Complementary, and Alternative MedicineDocument119 pagesIntegrative, Complementary, and Alternative Medicineشاجوان عباس محمد احمدNo ratings yet
- ComprehensionDocument38 pagesComprehensionkhantasleema196No ratings yet
- Health 11 Chapter 17 Alternative Medicine PDFDocument31 pagesHealth 11 Chapter 17 Alternative Medicine PDFdanicaNo ratings yet
- Communication and Collatboration Acute Care Mid-Point Reflective JournalDocument3 pagesCommunication and Collatboration Acute Care Mid-Point Reflective Journalapi-346386230No ratings yet
- HomeoDocument18 pagesHomeoMaheen HayatNo ratings yet
- Schizoaffective With Bipolar DisordersDocument14 pagesSchizoaffective With Bipolar DisordersNaomi MasudaNo ratings yet
- Ilovepdf MergedDocument125 pagesIlovepdf MergedinnyNo ratings yet
- Journal ReadingDocument8 pagesJournal ReadingKian HerreraNo ratings yet
- No ApprovedDocument154 pagesNo ApprovedAnnaNo ratings yet
- Research TBL Written ReportDocument6 pagesResearch TBL Written ReportChaze WaldenNo ratings yet
- Tni Intro PageDocument4 pagesTni Intro Pageapi-529216367No ratings yet
- Animal-Assisted - Therapy EvaluationDocument4 pagesAnimal-Assisted - Therapy Evaluationboriskei21No ratings yet
- Homoeopathy PosologyDocument27 pagesHomoeopathy PosologyLakshmi Deepak INo ratings yet
- Comfort and Hope in The Preanesthesia Stage in Patients Undergoing SurgeryDocument8 pagesComfort and Hope in The Preanesthesia Stage in Patients Undergoing SurgeryJohana CifuentesNo ratings yet
- Acupoint Stimulation On Weight Reduction For Obesity: A Randomized Sham-Controlled StudyDocument14 pagesAcupoint Stimulation On Weight Reduction For Obesity: A Randomized Sham-Controlled StudyMaurocoNo ratings yet
- Effectiveness of Complementary and Self-Help Treatments For Anxiety DisordersDocument18 pagesEffectiveness of Complementary and Self-Help Treatments For Anxiety DisordersSoulyogaNo ratings yet
- Address The Question Listed Below:: Kristen Nii-Jensen Weekly Evaluation 4 Fall 2014 NURS 260Document2 pagesAddress The Question Listed Below:: Kristen Nii-Jensen Weekly Evaluation 4 Fall 2014 NURS 260api-273463788No ratings yet
- The Impact of Multidisciplinary Rehabilitation On The Quality of Life of Hemodialysis Patients in IranDocument7 pagesThe Impact of Multidisciplinary Rehabilitation On The Quality of Life of Hemodialysis Patients in IranDewi KusumastutiNo ratings yet
- Seligman Consumer Reports StudyDocument16 pagesSeligman Consumer Reports StudyZara KhanNo ratings yet
- NguyenaDocument10 pagesNguyenaapi-699842071No ratings yet
- Am Vs PMDocument2 pagesAm Vs PMMohammed AlomarNo ratings yet
- Health Seeking BehaviourDocument22 pagesHealth Seeking Behaviour6ydn87w6gpNo ratings yet
- Adaptive QuizzingDocument166 pagesAdaptive QuizzingHuawei Li100% (1)
- History of Toxicity TestingDocument4 pagesHistory of Toxicity TestingIja Nur100% (1)
- By Trevor Hiltbrand - Reviwed By: Free Shipping On Orders $150+ (U.S. Only.)Document4 pagesBy Trevor Hiltbrand - Reviwed By: Free Shipping On Orders $150+ (U.S. Only.)Bruno ManestarNo ratings yet
- Date/Hour of Shift Focus Note ProgressDocument2 pagesDate/Hour of Shift Focus Note Progressako at ang exoNo ratings yet
- Boiron - DDR 2018 - GB PDFDocument218 pagesBoiron - DDR 2018 - GB PDFRicardoMadeiraNo ratings yet
- Crown & Bridge RemovalDocument1 pageCrown & Bridge RemovalCamilaNo ratings yet
- Can Cognitive Therapy Be Conducted by Computers. Eells, Barrett, Wrigh, ThaseDocument7 pagesCan Cognitive Therapy Be Conducted by Computers. Eells, Barrett, Wrigh, ThaseAylin Lidsay Feria GNo ratings yet
- DKD DR RatnaDocument29 pagesDKD DR Ratnaxiongmao2389No ratings yet
- Nursing Research: Unit 1Document8 pagesNursing Research: Unit 1KiranNo ratings yet
- Altitude SicknessDocument8 pagesAltitude SicknessAmira SalasNo ratings yet
- US - Recovery - Article - 2021Document8 pagesUS - Recovery - Article - 2021Odett NuñezNo ratings yet
- Intro To MED-SurgDocument47 pagesIntro To MED-SurgMarites Santos AquinoNo ratings yet
- Emotion-Focused Family Therapy (A Transdiagnostic Model For Caregiver-Focused Interventions)Document245 pagesEmotion-Focused Family Therapy (A Transdiagnostic Model For Caregiver-Focused Interventions)danivillavicencio917No ratings yet
- Student Copy Conti - Intrapartal Week8Document25 pagesStudent Copy Conti - Intrapartal Week8Toyour EternityNo ratings yet
- Detection of NoncomplianceDocument12 pagesDetection of Noncompliancesomayya waliNo ratings yet
- Power of Plants 1Document14 pagesPower of Plants 1api-399048965No ratings yet
- Letter of Support Template - 0 PDFDocument2 pagesLetter of Support Template - 0 PDFselamitspNo ratings yet
- San Antonio and Bexar County Petition For TRO Against Gov. Abbott Over Local Control of COVID-19 Prevention EffortsDocument29 pagesSan Antonio and Bexar County Petition For TRO Against Gov. Abbott Over Local Control of COVID-19 Prevention EffortsKENS 5No ratings yet
- HSE Commitment and Policy?Document6 pagesHSE Commitment and Policy?radhesrikrishnaNo ratings yet
- CL489F Physiotherapy Reassessment ReportDocument4 pagesCL489F Physiotherapy Reassessment ReportnaeemullahNo ratings yet
- Family DeclarationDocument5 pagesFamily DeclarationVanlal MuanpuiaNo ratings yet
- Part-IDocument507 pagesPart-INaan SivananthamNo ratings yet
- SS20120100010 42491076 PDFDocument6 pagesSS20120100010 42491076 PDFRishiraj JaiswalNo ratings yet
- UntitledDocument22 pagesUntitledBenjamin Swami100% (7)
- Nepheline Syenite - Various Grades (A200-A270)Document5 pagesNepheline Syenite - Various Grades (A200-A270)Lynne MarrNo ratings yet
- EO-GA-32 Continued Response To COVID-19 IMAGE 10-07-2020Document7 pagesEO-GA-32 Continued Response To COVID-19 IMAGE 10-07-2020Jakob RodriguezNo ratings yet
- Bronchopneumonia Care PlanDocument6 pagesBronchopneumonia Care PlanAbhijit Soundade0% (1)
- Nominal Roll N035 1bnm10jDocument2 pagesNominal Roll N035 1bnm10jsubiNo ratings yet
- 1 TTP Mozambique - STE EIS Vol - I - Submitted - For - Disclosure - 0Document227 pages1 TTP Mozambique - STE EIS Vol - I - Submitted - For - Disclosure - 0Luciana MirandaNo ratings yet
- Format Nutritional StatusDocument43 pagesFormat Nutritional StatusDirkie Meteoro Rufin83% (6)
- Chapter 2Document15 pagesChapter 2Daniel John LingamenNo ratings yet