21/02/2011
Interface and colloidal science-Colloidal stability
Colloidal Stability
• Particles collide due to the Brownian motions (random) or convection or sedimentation.
• The rate of aggregation is in general determined by the frequency of collisions and the
probability of cohesion during collision.
• The probability of cohesion depends on the chemical interactions acting among the
particles.
• These interactions can be tuned to increase the colloidal stability.
BUT ….
Thermodynamics fights against us!
Thermodynamically the particles tend to aggregate
Interface and Colloidal Science 1
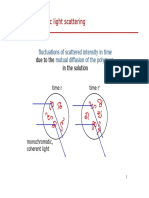
Stability of colloidal solutions- Ostwald ripening
In colloidal solutions a barrier of 15-25 kT is sufficient to give colloid stability (with a
Debye length > 20 nm)
However the system is not THERMODYNAMICALLY stable
Over time solid or liquid solutions change their inhomogeneous structure: over time
the emulsions may coarsen in size and small particles aggregate into larger ones.
This growth is called Oswald ripening
Larger crystals are more energetically favored than smaller crystals
Small crystals have a larger surface area to volume ratio than large crystals.
Molecules on the surface are energetically less stable than the ones already well
ordered and packed in the interior
MonteCarlo numerical simulation of the Ostwald ripening
An everyday example is the re-crystallization of water within ice cream which gives
old ice cream a gritty, crunchy texture.
Interface and Colloidal Science 2
1
� 21/02/2011
Interface and colloidal science-Surface modification
Surface modifications
The modification of the surface changes the surface properties
Higher contact angle lower wetting
Wetting effects play a major role in
microfluidics.
Application examples from daily life range from
diapers over ink jet printers to the never-dripping
outlet of a tea pot.
From University of Erlangen (D)
http://www.youtube.com/watch?v=agiTkQyD43M
Interface and Colloidal Science 3
Interface and colloidal science-Surface modification
Surface modifications Hydrophobic
Superhydrophobic coatings polymer
Increase of the contact angle
http://www.youtube.com/watch?v=riXp_Q-
fDv8&feature=player_detailpage http://wn.com/superhydrophobic
University of Missouri College of Engineering
Interface and Colloidal Science 4
2
� 21/02/2011
Stability of colloidal solutions-Effect of Polymers
Adding polymers to the colloidal suspension is a way to stabilize it.
Polymers can be adsorbed or chemically attached to the particle.
Limitations of charge stabilization
•Use of polar solvent
•Very sensible to addition of salt (high valency counter-ion)
•The suspension are only stabilize KINETICALLY (problems with high concentrations
or under shear
The addition of polymers can provide three different type of stabilization
•Steric stabilization: polymer is adsorbed or chemically attached to the surface of
the particle (particle repulsion)
•Depletion interactions: where non-adsorbing polymers in solution induce
attraction
•Bridging interactions: where adsorbing polymers induce particle attraction by
adsorbing to two particles at the same time
Interface and Colloidal Science 5
Introduction to polymer physics
• Macromolecules MW = 103 – 106 g/mol
• Repeat units
• 1 type: homopolymer
• A-A-A-A-A
• few types: copolymer
• A-B-B-A-B-B-B
monomer
• many types: e.g. proteins
• Sequence macromolecule
• Connectivity, topology: linear, branched, comb, star, ring,
Interface and Colloidal Science 6
3
� 21/02/2011
Introduction to polymer physics-Molecular weight
Due to the high Mw and the high number of chains all the properties a polymeric
material are expressed in term of averaged properties
Molar mass distribution
In the case of Mw the weighted averages can be taken with the weight fraction or the
mole fraction:
Mn: Number average molecular weight
N M
N i i
Mn i
i
M i n i M i
N N
i
i i
i
i
i
Can be determined by gel permeation chromatography or viscosimetry
Mw: Weight average molecular weight
N M i
2
i
Mn i
N Mi
i i
Interface and Colloidal Science 7
Introduction to polymer physics-Molecular weight
• Polydispersity index
Mn / Mw=1 for monodisperse polymers
Example:
MW = 1000 (20%), MW = 10 000 (60 %), MW = 100 000 (20 %)
M n 0.2 1000 0.6 10000 0.2 100000 26200
M w 0.2 1000 2 0.6 10000 2 0.2 100000 2 78633
Interface and Colloidal Science 8
4
� 21/02/2011
Introduction to polymer physics-Polymer interactions
Entanglements and effect of the Mw on dynamic properties (viscosity)
Polymers can physically entangle. This property is Mw dependent.
Non-Newtonian
behaviour
Newtonian
behaviour
η M for M M c
η M 3.4 for M M c
Interface and Colloidal Science 9
Introduction to polymer physics- Radius of gyration
Radius of gyration
Structural property that describes the distribution of masses and give an
indication of the size of an object.
It is calculated as the root mean square distance of the objects' parts from its
center of gravity
N
r r
For a polymer chain: 1
Rg2
2
i m
N i 1
1 N
rm
N
r
i 1
i
For polymer chain with the same Mw
More expanded chain larger Rg
More coiled chain smaller Rg
Interface and Colloidal Science 10
5
� 21/02/2011
Introduction to polymer physics-Polymer solutions
In solution the conformation of the single polymer chain depends on the solvent.
Good solvent: the chain is more expanded and try
to maximize the number of contacts with the fluid.
Bad solvent the chain segments stay close to each
other and the polymer chain is packed to limit the
polymer-fluid contacts. In the limit of a very bad
solvent the polymer chain merely collapses to form a
hard sphere.
The radius of gyration scales with the number of polymer segments
R g Nυ
For a good solvent n=3/5; For a bad solvent n=1/3; for q solvent n=1/3
Interface and Colloidal Science 11
Introduction to polymer physics-Polymer solutions
Ideal Chains
In bulk, polymers behave like ideal chains: the polymer chain can be
described as a random walk (no excluded volume). Any monomer interaction
is neglected.
N number of monomers
L: length of the monomer
The energy of the polymer is taken to be independent of its shape, which means
that at the thermodynamic equilibrium, all of its shape configurations are equally
likely to occur as the polymer fluctuates in time, according to the Maxwell-
Boltzmann distribution.
Average end-to-end distance and relation with the radius of gyration
2 R2
R Nl R g2
6
Interface and Colloidal Science 12
6
� 21/02/2011
Introduction to polymer physics-Polymer solutions
Theta (q) solvent, q temperature
In solution the polymer chain behaves like a real chain (with excluded volume
taken into account): self avoiding walk.
but ..
at the theta conditions the chain behaves like an ideal chain.
The polymer-solvent (positive or negative) and polymer-polymer interactions are
counterbalanced.
Each solvent has a specific temperature (theta) at which the polymer chain
behaves like an ideal chain.
Interface and Colloidal Science 13
Introduction to polymer physics-Polymer interactions
Polymer interactions and parameter: Mixing polymers
The conformation of polymer chains in solution depends on their architecture and solvent
quality. Flory and Huggins developed a theory for the solubility of polymers that predicts the
free energy of mixing from the polymer and solvent volume fractions and the net solvent-
polymer interaction energy (the (Flory) parameter).
p
ΔA m k BTN s ln s ln p s p
rs rp
The mixing depends on the
Negative entropic contribution
value of parameter
i volume fraction
N number density of molecules
ri chain length
Interface and Colloidal Science 14
7
� 21/02/2011
Introduction to polymer physics-Polymer interactions
Polymer interactions and parameter: Polymer at the interface
In a similar way we can define a Flory surface parameter s
s u2 s u1s u11 u22 k BT
1
2
uij: pairwise contact energy normally attractive (<0)
Adsorption occurs when s is
larger than a critical value that
takes into account the fact that
when the polymer is adsorbed
the total entropy of the system
(polymer+solvent) decrease
(penalty in the configurational
entropy)
Interface and Colloidal Science 15
Introduction to polymer physics-Polymer interactions
Copolymers and amphiphilic molecules
When the polymeric chain is formed by different monomeric species
Block copolymer: AAAABBBBBAAAA
Random copolymer: ABBBAABBABAAB
Alternating: ABABABABA
Interface and Colloidal Science 16
8
� 21/02/2011
Stability of colloidal solutions-Effect of Polymers
Steric stabilization: polymer is adsorbed or chemically attached to the surface of the
particle (particle repulsion)
Cover the colloidal particle with a dense polymer layer (typically adsorbed from solution).
Steep repulsion between particles
Polymers at surface may be adsorbed or depleted depending of the value of the surface
s parameter
If s > sc adsorption; If s < sc depletion
Adsorption isotherm adsorbed amount per Polymer volume fraction () vs.
unit per surface vs. ceq (equilibrium distance from the surface (Z)
concentration of the polymer in solution)
Few mg/m2
Interface and Colloidal Science 17
Stability of colloidal solutions-Effect of Polymers
Steric stabilization
Polymers can be either chemically attached or physically adsorbed.
Constant polymer volume fraction j
L0
The polymer Concentration of the
concentration is D=2L0
polymer increases
doubble
Same concentration value
as initially in the gap
Interface and Colloidal Science 18
9
� 21/02/2011
Stability of colloidal solutions-Effect of Polymers
Steric stabilization
In a good solvent the local increase in polymer concentration carries a free energy
penalty and then a repulsion steric interaction between particles
The repulsion is felt as soon as D < 2L0 (D: surface separation; L0: unperturbed layer
thickness) and increases steeply as D< L0
An analytical expression for the steric interaction (Vster) says that it scales as the
squared of the surface coverage
Vster2
Importance of the high surface coverage of an effective stabilization
Van der Waals attractions still operate between colloidal particles but stability can be
achieved if they are weak at D≈L0
Interface and Colloidal Science 19
Stability of colloidal solutions-Effect of Polymers
Steric stabilization
PEO(Rg=13 nm) is adsorbed in onto mica surface
PEO: -(-CH2-CH2-O-)n-
Force much less:
Slow adjustment
Compression
of the polymer Compression after decompression
Repulsion D≈ 6Rg
conformation
The polymer
chains have to
be in a well
relaxed and
stable
conformation
(importance of
solvent and
temperature)
Interface and Colloidal Science 20
10
� 21/02/2011
Stability of colloidal solutions-Effect of Polymers
Steric stabiliser design
•High surface coverage
•Strong adsorption (s > sc )
•Good solvent for stability of the chain ( < 0.5)
•Low free polymer concentration (Ceq≈ 0)
The adsorption needs to be at the plateau region of the isotherm
Low free polymer is to avoid depletion attraction
Copolymers usually more efficient then homopolymers
(A would absorb and B will stick out into the solution and provide steric
stabilization)
Interface and Colloidal Science 21
Stability of colloidal solutions-Effect of Polymers
Depletion Interactions: addition of free non-adsorbing polymer in solution
Polymer chains occupy a volume whose value depends on their conformation and they
interact with the colloidal particles as hard spheres (excluded volume).
In q solvent -> polymer coils can be approximated as a sphere of diameter 2L0
Polymer coils are excluded from a depletion layer of L0 thickness around each particle
When two particles approach to a separation less than 2L0, the depletion layer overlap
and the free volume for the polymer increase.
Due the polymer osmotic pressure an effective attraction results between the particles
Interface and Colloidal Science 22
11
� 21/02/2011
Stability of colloidal solutions-Effect of Polymers
Depletion Interactions
Vster-P
Vdep: depletion potential
x: rpol/rcolloid
r/d: particle separation/rcol
Smaller polymer narrow
attraction region
Depletion mechanism allows for switching on particle attractions in a very controlled
manner
Interface and Colloidal Science 23
Stability of colloidal solutions-Effect of Polymers
Bridging interactions: adsorbing polymers induce particle attraction by adsorbing to
two particles at the same time
At low (surface coverage) a polymer chain may attach to more than one colloidal
particle.
This can lead to attraction
The bridging interactions can be used to maximize colloidal aggregation (polymeric
flocculants)
Interface and Colloidal Science 24
12