Professional Documents
Culture Documents
Protons, Neutrons, and Electrons Practice Worksheet
Uploaded by
crisanto cabatbatCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Protons, Neutrons, and Electrons Practice Worksheet
Uploaded by
crisanto cabatbatCopyright:
Available Formats
Protons, Neutrons, and Electrons Practice Worksheet
Helpful Concepts:
# Protons + # Neutrons = Atomic mass # (this is usually shown as an average for all isotopes of an element)
# Protons = Atomic Number
# Electrons = Protons (atoms of an element are electrically neutral so + = – )
# Neutrons = Atomic Mass – Atomic Number OR Atomic Mass – # of Protons
# of Valence Electrons = Representative Group # (Roman Numeral A’s Group #; transition metals require research)
# of Electron Energy Levels = Period #
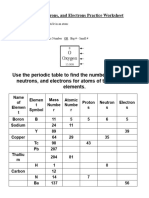
Use a periodic table to find the numbers of protons, neutrons, electrons, etc. for atoms of the following elements.
Name of Element Atomic Valence Electron
Element Symbol Mass Number Protons Neutrons Electrons Electrons Energy
Number Levels
Boron B 11 5 5 6 5
Sodium 24 11
Y 89 39
Copper 29 35
Tc 98 43
Pb 207
Thallium 204 81
H 0
Carbon 12
N 7
Ba 56
Calcium
Si 14
Argon 18
Mg 12 12
You might also like
- Subatomic Particles WsDocument1 pageSubatomic Particles WsYhena ChanNo ratings yet
- Protons, Neutrons, and Electrons Practice Worksheet For 8th GradeDocument2 pagesProtons, Neutrons, and Electrons Practice Worksheet For 8th GradeDrama Music67% (3)
- Subatomic Particles -FILLDocument2 pagesSubatomic Particles -FILLALMERA SHELLA CABOGONo ratings yet
- Protons, Neutrons, and Electrons Practice WorksheetDocument1 pageProtons, Neutrons, and Electrons Practice WorksheetMelerose Dela SernaNo ratings yet
- Protons, Neutrons, and Electrons Practice Worksheet For 8th Grade AnswersDocument2 pagesProtons, Neutrons, and Electrons Practice Worksheet For 8th Grade AnswersDrama Music92% (13)
- Subatomic Particles WsDocument1 pageSubatomic Particles WsJessa FerrerNo ratings yet
- Protons, Neutrons, and Electrons Practice WorksheetDocument1 pageProtons, Neutrons, and Electrons Practice WorksheetRosa SaritaNo ratings yet
- Atomic Mass and Atomic Number WorksheetDocument1 pageAtomic Mass and Atomic Number WorksheetGuayNo ratings yet
- Basic Atomic Structure Worksheet ANSWERSDocument2 pagesBasic Atomic Structure Worksheet ANSWERSMiss RonaNo ratings yet
- 118 ElementsDocument1 page118 Elementsqwerty100% (1)
- 1 Atomic Structure and Ions AnswersDocument2 pages1 Atomic Structure and Ions AnswersAwais NaeemNo ratings yet
- Basic Atomic Structure Worksheet ANSWERSDocument2 pagesBasic Atomic Structure Worksheet ANSWERSlex marantalNo ratings yet
- Elements and The Periodic Table WorksheetDocument4 pagesElements and The Periodic Table WorksheetVictoria StewartsonNo ratings yet
- Atomic Mass and Atomic Number Worksheet KeyDocument1 pageAtomic Mass and Atomic Number Worksheet KeyRalphNacis0% (1)
- Eliza Budarz - B6.1 Why Are Metals Useful.Document11 pagesEliza Budarz - B6.1 Why Are Metals Useful.Eliza BudarzNo ratings yet
- 118 Elements and Their Symbols and Atomic Numbers TableDocument1 page118 Elements and Their Symbols and Atomic Numbers TableAung ThawNo ratings yet
- Atoms and Ions Worksheet AnswersDocument1 pageAtoms and Ions Worksheet AnswersFrancis Olila0% (1)
- Periodictable Dave Lo PDFDocument2 pagesPeriodictable Dave Lo PDFRafaela DavidNo ratings yet
- Wide World of Minerals Calendar 2024Document32 pagesWide World of Minerals Calendar 2024achint GoelNo ratings yet
- Periodic Table of the Elements: Key Elements at a GlanceDocument2 pagesPeriodic Table of the Elements: Key Elements at a GlanceIan RiveraNo ratings yet
- IGCSE Periodic Table v2Document1 pageIGCSE Periodic Table v2Umar ElèvénNo ratings yet
- Solid State PhysicsDocument417 pagesSolid State Physicsapi-377220450% (2)
- Preparation of Reagents of Desired StrengthDocument4 pagesPreparation of Reagents of Desired Strengthapi-3803371No ratings yet
- CHEM SPM Periodic Table BWDocument1 pageCHEM SPM Periodic Table BWangie081250% (2)
- PERIODIC TABLE TITLEDocument1 pagePERIODIC TABLE TITLElingarajugowdaNo ratings yet
- The Periodic Table C PDFDocument177 pagesThe Periodic Table C PDFmarius1966No ratings yet
- 7.1 Atomic Number and Mass NumberDocument3 pages7.1 Atomic Number and Mass NumberMuzammil HassanNo ratings yet
- THE PERIODIC TABLE OF THE ELEMENTSDocument1 pageTHE PERIODIC TABLE OF THE ELEMENTSfocuc98No ratings yet
- Atomic MassDocument1 pageAtomic MassDeepti JainNo ratings yet
- Handout 4 Chemistry Periodic TableDocument3 pagesHandout 4 Chemistry Periodic TableZëky NhächëngöNo ratings yet
- First 20 Elements 2022 - Sheet1Document1 pageFirst 20 Elements 2022 - Sheet1shintaro midorimaNo ratings yet
- IGCSE Periodic Table v2Document1 pageIGCSE Periodic Table v2j2zttgtwfh100% (1)
- Atomic Structure Practice Name - : (Atomic Mass-Atomic Number) (Same As Number of Protons)Document1 pageAtomic Structure Practice Name - : (Atomic Mass-Atomic Number) (Same As Number of Protons);No ratings yet
- Ion Worksheet KEYDocument1 pageIon Worksheet KEYAna Marie Corales TabunarNo ratings yet
- Gmelin Handbook of Inorganic Chemistry V08a 1985 PDFDocument304 pagesGmelin Handbook of Inorganic Chemistry V08a 1985 PDFLeonardo Sotelo MontañaNo ratings yet
- All About The Periodic Table - Home Laboratory WorksheetDocument4 pagesAll About The Periodic Table - Home Laboratory WorksheetFrank Ed SerranoNo ratings yet
- Ionic Radius - Wikipedia PDFDocument29 pagesIonic Radius - Wikipedia PDFடேவிட் ஸ்No ratings yet
- Periodic Table V1.0Document10 pagesPeriodic Table V1.0EyeoSkyNo ratings yet
- Chemistry Holiday HomeworkDocument5 pagesChemistry Holiday HomeworkRavibabu BoddaNo ratings yet
- CH 4Document6 pagesCH 4SujalNo ratings yet
- Tadashi Okuyama, Mark Maskill - Organic Chemistry - A Mechanistic Approach-Oxford University Press (2013)Document681 pagesTadashi Okuyama, Mark Maskill - Organic Chemistry - A Mechanistic Approach-Oxford University Press (2013)Sooraj Srinivasan100% (13)
- Subatomic Particle IdentificationDocument1 pageSubatomic Particle IdentificationDenise AcunaNo ratings yet
- Annotated-Atomic Structure Bohr Models-1Document2 pagesAnnotated-Atomic Structure Bohr Models-1Ivania Joselina Lobo MontoyaNo ratings yet
- Atomic Particles Chart: + o - ST ND RD THDocument2 pagesAtomic Particles Chart: + o - ST ND RD THZexdxrNo ratings yet
- Periodic Table ColorDocument1 pagePeriodic Table Colorapi-619044126No ratings yet
- List of Elements W/Che Mical Group Block: CR KRDocument3 pagesList of Elements W/Che Mical Group Block: CR KRMark Nathan GarciaNo ratings yet
- The volume of one mole of any gas is 24 dm3 at room temperature and pressureDocument1 pageThe volume of one mole of any gas is 24 dm3 at room temperature and pressureGrape100% (1)
- Classifying Periodic Table of Elements-ActiviDocument1 pageClassifying Periodic Table of Elements-ActiviKimberly Joy LungayNo ratings yet
- Tabel KimiaDocument1 pageTabel Kimiafairy kaaguraaNo ratings yet
- List of ElementsDocument2 pagesList of ElementsKathleen Glyze CarcasonaNo ratings yet
- Chemistry P - 2Document17 pagesChemistry P - 2shezin rahmanNo ratings yet
- Acs Periodic Table Poster - DownloadDocument1 pageAcs Periodic Table Poster - DownloadBenedick CruzNo ratings yet
- Atomic Number and Atomic MassDocument2 pagesAtomic Number and Atomic MassKayra KamberogluNo ratings yet
- KCSE Form 2 NotesDocument139 pagesKCSE Form 2 NotesN KatanaNo ratings yet
- ChemistryDocument3 pagesChemistryMurphy_AMDNo ratings yet
- Periodic Table Downloadable Version BookletDocument2 pagesPeriodic Table Downloadable Version BookletAna HakuraNo ratings yet
- The Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 40 to EN 363From EverandThe Mechanical and Physical Properties of the British Standard EN Steels (B.S. 970 - 1955): EN 40 to EN 363No ratings yet
- The Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyFrom EverandThe Uniqueness of Biological Materials: International Series of Monographs in Pure and Applied Biology: ZoologyNo ratings yet
- Analysis of the New Metals: Titanium, Zirconium, Hafnium, Niobium, Tantalum, Tungsten and Their AlloysFrom EverandAnalysis of the New Metals: Titanium, Zirconium, Hafnium, Niobium, Tantalum, Tungsten and Their AlloysNo ratings yet
- Presentation STIs GRade 9Document9 pagesPresentation STIs GRade 9crisanto cabatbatNo ratings yet
- Academic Excellence Award 2nd QTRDocument4 pagesAcademic Excellence Award 2nd QTRcrisanto cabatbatNo ratings yet
- Module 2 - Changes in MatterDocument27 pagesModule 2 - Changes in Mattercrisanto cabatbatNo ratings yet
- IS Project 5 - (LRAZONA)Document4 pagesIS Project 5 - (LRAZONA)crisanto cabatbatNo ratings yet
- Untitled DesignDocument25 pagesUntitled Designcrisanto cabatbatNo ratings yet