Professional Documents
Culture Documents
Moore2012 9
Uploaded by
Rahman Spn0 ratings0% found this document useful (0 votes)
2 views1 pageOriginal Title
moore2012-9
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
2 views1 pageMoore2012 9
Uploaded by
Rahman SpnCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
44 T.A.
Moore / International Journal of Coal Geology 101 (2012) 36–81
upwards through multiple seams (Faiz et al., 2007a). Furthermore, in
the Bulli seam within the same basin, ethane proportions vary signifi-
cantly in relation to the depth of the coal seam (Faiz et al., 2003).
Creedy and Pritchard (1983) and Rice (1993) also found a relationship
between depth and gas quality, though this doesn't appear to be univer-
sal across basins. An interesting association in the San Juan Basin exists
where coals seams below the topographically higher recharge areas
have the highest CH4 concentrations (and importantly low CO2; Scott
et al., 1994). This has been postulated to be a result of water differentially
flushing the more mobile CO2 down dip, effectively concentrating the
CH4 (Cui et al., 2004). Some production companies do note that CO2
will increase at the expense of CH4 with continued degassing of the
seam; in part this might be the result of different desorption kinetics
between CO2 and CH4 (Cui et al., 2004).
Care does have to be taken when analysing for gas quality as some
gases may be introduced or created during the sampling process or
even be altered from the containment vessel itself (van Holst et al.,
2010). For example, if canisters are not constructed out of material
which are inert and non reactive, it has been shown that significant
H2 may be produced (Faraj and Hatch, 2004). Nitrogen can also be
an introduced contaminant (as part of atmospheric air) during gas
quality analysis (Kim 1973). There is no doubt that N2 exists naturally
within some higher rank coals (Creedy and Pritchard, 1983; Hunt,
1979) but in some cases O2 may react with the coal itself and be pref-
erentially adsorbed, with the effect of leaving excess N2 in the gas
mixture (Creedy and Pritchard, 1983; Havton, 2003; Jin et al., 2010).
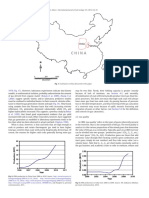
Fig. 13. Timing of gas type generation in relation to temperature for humic type organic It is important that this relic N2 is not mistaken as being inherent
matter. Also showing approximate H/C ratios with burial stages. Modified from Hunt within the seam.
(1979) and Zhang et al. (2008).
3.4. Desorption
things go wrongly, through use of bacterially contaminated drilling
or fracturing fluids introduced into the reservoir; in the latter case, The amount of actual gas (also referred to as ‘gas charge’) in a coal
cleaning up the reservoir can be time consuming and expensive. bed can be estimated through taking a sample of fresh coal and
In general, the gases found within a CBM reservoir are greatly desorbing gas from it over a period of time. When a sample of coal is
influenced by rank (see Section 4.1). Because of the nature of biogenic brought to the surface and placed in a canister some of the gas will be
processes, low-rank coals contain mostly CH4 and usually only small released from the coal. At depth, reservoir pressure, which is mainly
quantities of CO2. However, in some plays there may be spatial variation from hydrostatic head, keeps gas molecules physically adsorbed onto
in CH4 and CO2 because of the kinetics of differential gas transport. This the surface of the coal micropores (see also Section 5.2 and Busch and
variation can have economic ramifications over the production life of a Gensterblum, 2011 for more detail on sorption properties). The zero
basin (Cui et al., 2004). hydrostatic pressure at the Earth's surface allows the gas to desorb out
Some CBM plays may have a multitude of gases present whose of the coal and thus be measured. This process is called desorption.
relative concentrations vary greatly laterally and vertically within the Some procedures dictate that desorption should take place at the equiv-
basin. For example, in the Sydney basin CO2 concentrations increase alent reservoir temperature that the sample came from (e.g. American
Standard Testing Material [ASTM] D7965-10, 2010) whilst other proce-
dures place less emphasis on that requirement, relying more on math-
ematical corrections to simulate reservoir temperature (e.g. AS 3980–
1999, Anonymous, 1999; Saghafi et al., 1995; Williams, 1996).
Some service companies are now offering in situ gas measurements
that determine the critical desorption pressure (CDP) without the need
for core. As this technology is just being commercially trialled, the reader
is directed to www.welldog.com and www.weatherfords.com for addi-
tional information on these procedures. See also Lamarre and Pope
(2007) and Carlson et al. (2008) for a discussion. It is also possible to
use well cuttings for desorption (ASTM, 2010; Newell, 2007), though
these will yield less certain results.
Some of the earliest modern desorption procedures were developed
by the U.S. Bureau of Mines in the 1970s and early 1980s (e.g. Diamond,
1978; Diamond and Levine, 1981; Kim, 1973, 1977; Kim and Douglas,
1973; McCulloch et al., 1975). The basic concept is that coal samples
are brought quickly to surface (preferably using a wire-line coring
system), placed in a leak proof canister of roughly the same size as the
sample, and over a period of time the gas that is evolved is measured.
Since those early studies, there have been improvements (e.g. Barker
et al., 2002, Crosdale et al., 2005; McLennan et al., 1995; Moore and
Fig. 14. Changes in relative volumes of generated gases to vitrinite reflectance; from Butland, 2005; Stricker et al., 2006; Yee et al., 1993; also see Diamond
experimental pyrolysis data. Modified from Zhang et al. (2008). and Schatzel, 1998 for an earlier review), but the essential steps have
You might also like
- Pub - Essentials of Nuclear Medicine Imaging 5th Edition PDFDocument584 pagesPub - Essentials of Nuclear Medicine Imaging 5th Edition PDFNick Lariccia100% (1)
- 280 1013 1 PBDocument16 pages280 1013 1 PBZahia ZinebNo ratings yet
- Storage of CO2 As Hydrate in Depleted Gas Reservoirs-PA-PDocument11 pagesStorage of CO2 As Hydrate in Depleted Gas Reservoirs-PA-PBolsec14No ratings yet
- Moore2012 7Document1 pageMoore2012 7Rahman SpnNo ratings yet
- 10 1016@j Ijggc 2019 04 016Document23 pages10 1016@j Ijggc 2019 04 016kiingkangNo ratings yet
- International Journal of Greenhouse Gas ControlDocument20 pagesInternational Journal of Greenhouse Gas ControlgregNo ratings yet
- SPE 93773 CO Injection in CarbonatesDocument9 pagesSPE 93773 CO Injection in CarbonatesanderlethNo ratings yet
- Studies On The Adsorption Behavior of Co - CH Mixtures Using Activated CarbonDocument13 pagesStudies On The Adsorption Behavior of Co - CH Mixtures Using Activated CarbonyahyaNo ratings yet
- 7 Review PaperDocument17 pages7 Review PaperMarzhan ZhandildinaNo ratings yet
- CO2Storage Acad JourlDocument13 pagesCO2Storage Acad JourlgorleNo ratings yet
- s12182 021 00548 ZDocument13 pagess12182 021 00548 ZRamadhan KusumaNo ratings yet
- Study of LimestoneDocument5 pagesStudy of LimestoneAlberto Moreno GomezNo ratings yet
- Coal Bed Methane Reservoir Simulation StudyDocument16 pagesCoal Bed Methane Reservoir Simulation Studymohsin abroNo ratings yet
- Mienert2022 Chapter FindingAndUsingTheWorldSGasHydDocument20 pagesMienert2022 Chapter FindingAndUsingTheWorldSGasHydAlexander Peñaloza TinocoNo ratings yet
- 18BCL0273 VL2019201003765 DaDocument61 pages18BCL0273 VL2019201003765 DaAaditya AgrahariNo ratings yet
- Modeling CO2 Storage in Aquifers With A Fully-CoupDocument17 pagesModeling CO2 Storage in Aquifers With A Fully-CoupFatimah Az ZahraNo ratings yet
- Bowen Fields AustraliaDocument12 pagesBowen Fields AustraliaAdmirerNo ratings yet
- Ren Et Al (2019) Gas Storage Potential and Electrohydraulic Discharge (EHD) Stimulation of Coal Seam Interburden From The Surat BasinDocument13 pagesRen Et Al (2019) Gas Storage Potential and Electrohydraulic Discharge (EHD) Stimulation of Coal Seam Interburden From The Surat BasinFrancyNo ratings yet
- 1 s2.0 S0360544217314081 MainDocument17 pages1 s2.0 S0360544217314081 Maindorian.axel.ptNo ratings yet
- Modelling Co Solubility in Pure Water and Nacl-Type Waters From 0 To 300 8C and From 1 To 300 Bar Application To The Utsira Formation at SleipnerDocument13 pagesModelling Co Solubility in Pure Water and Nacl-Type Waters From 0 To 300 8C and From 1 To 300 Bar Application To The Utsira Formation at SleipnerzibaNo ratings yet
- Review of Gas Adsorption in Shales For Enhance - 2019 - Journal of Petroleum SciDocument10 pagesReview of Gas Adsorption in Shales For Enhance - 2019 - Journal of Petroleum Scihenrique lopesNo ratings yet
- Oxygenation of Atmosphere and OceansDocument13 pagesOxygenation of Atmosphere and OceansUfiNo ratings yet
- Shams Kalam 2020 - Carbon Dioxide Sequestration in Underground FormationsDocument23 pagesShams Kalam 2020 - Carbon Dioxide Sequestration in Underground FormationsJose Luis Gonzalez TolosaNo ratings yet
- New Directions in Black Carbon Organic Geochemistry: C.A. MasielloDocument13 pagesNew Directions in Black Carbon Organic Geochemistry: C.A. MasielloSardar SaleemNo ratings yet
- Royer 2006Document11 pagesRoyer 2006JanNo ratings yet
- CO2 Sequestration in Subsurface Saline AquifersDocument47 pagesCO2 Sequestration in Subsurface Saline Aquifersmoscow01No ratings yet
- 1-S2.0-0008884687900123-Main - Pdf-Ho and Lewis PDFDocument16 pages1-S2.0-0008884687900123-Main - Pdf-Ho and Lewis PDFPriscilla CordeiroNo ratings yet
- Journal of Petroleum Science and EngineeringDocument24 pagesJournal of Petroleum Science and Engineeringkhuzestan nanonamaNo ratings yet
- 1 s2.0 S2096249517300273 MainDocument17 pages1 s2.0 S2096249517300273 Maindorian.axel.ptNo ratings yet
- Global Biogeochemical Cycles - 2006 - Aumont - Globalizing Results From Ocean in Situ Iron Fertilization StudiesDocument15 pagesGlobal Biogeochemical Cycles - 2006 - Aumont - Globalizing Results From Ocean in Situ Iron Fertilization Studiesleon.jogos.55No ratings yet
- Berger Et Al 1989 - Ocean Productivity and PaleoproductivityDocument19 pagesBerger Et Al 1989 - Ocean Productivity and PaleoproductivityYaimaNo ratings yet
- Per Era 2015Document19 pagesPer Era 2015Nilesh SinghalNo ratings yet
- A Review On Carbonation of Concrete and Its Predictive ModellingDocument16 pagesA Review On Carbonation of Concrete and Its Predictive ModellingnagarajuNo ratings yet
- 1reactions Between Olivine and Co2 Rich Seawater at 300 C Implications For h2 Generation and Co2 Sequestration On The Early EarthDocument10 pages1reactions Between Olivine and Co2 Rich Seawater at 300 C Implications For h2 Generation and Co2 Sequestration On The Early EarthLUCIA BEATRICE NECHIFOR GRIGORENo ratings yet
- COsub2sub Geological Sequestration in Heterogeneous Binary Media Effects of Geological and Operational Conditionsadvances in GeoEnergy ResearchDocument14 pagesCOsub2sub Geological Sequestration in Heterogeneous Binary Media Effects of Geological and Operational Conditionsadvances in GeoEnergy ResearchJoseph IRANZINo ratings yet
- Criteria For Intermediate Storage of Carbon Dioxide in Geological FormationsDocument11 pagesCriteria For Intermediate Storage of Carbon Dioxide in Geological Formationsshen shenNo ratings yet
- Journal of Natural Gas Science and EngineeringDocument8 pagesJournal of Natural Gas Science and EngineeringLOLA PATRICIA MORALES DE LA CUBANo ratings yet
- Structural Effects On The High Temperature Adsorption of CO2 On A Synthetic HydrotalciteDocument9 pagesStructural Effects On The High Temperature Adsorption of CO2 On A Synthetic HydrotalciteMahmood KhanNo ratings yet
- Chemical Engineering Science: Shih-Chieh Hsu, Chungsying Lu, Fengsheng Su, Wanting Zeng, Wenfa ChenDocument8 pagesChemical Engineering Science: Shih-Chieh Hsu, Chungsying Lu, Fengsheng Su, Wanting Zeng, Wenfa Chendevil cryNo ratings yet
- Chemosphere: Changlin Zhan, Yongming Han, Junji Cao, Chong Wei, Jiaquan Zhang, Zhisheng AnDocument9 pagesChemosphere: Changlin Zhan, Yongming Han, Junji Cao, Chong Wei, Jiaquan Zhang, Zhisheng AnUsman AliNo ratings yet
- Pipeline Gas Hydrate Formation and Treatment A ReviewDocument12 pagesPipeline Gas Hydrate Formation and Treatment A ReviewAlex AlexNo ratings yet
- 1 s2.0 S1750583608000066 MainDocument20 pages1 s2.0 S1750583608000066 MaingregNo ratings yet
- Role of Induction Time On Carbon Dioxide and Methane Gas Hydrate KineticsDocument9 pagesRole of Induction Time On Carbon Dioxide and Methane Gas Hydrate KineticsJAI SAHITHNo ratings yet
- Zhang 2011Document13 pagesZhang 2011Gabriel EvanNo ratings yet
- International Journal of Mineral Processing: Ali Vazirizadeh, Jocelyn Bouchard, Yun ChenDocument11 pagesInternational Journal of Mineral Processing: Ali Vazirizadeh, Jocelyn Bouchard, Yun ChenrajuvadlakondaNo ratings yet
- Sim of CO2 Stor in Saline AqsDocument29 pagesSim of CO2 Stor in Saline AqsFatimah Az ZahraNo ratings yet
- Paper Kapsel BDocument7 pagesPaper Kapsel BAlipAlBashriNo ratings yet
- P2.Hydrogen Adsorption Desorption inDocument3 pagesP2.Hydrogen Adsorption Desorption inD. SilambarasanNo ratings yet
- SPE Co2Document28 pagesSPE Co2Gabriel Alexander Garcia VacaNo ratings yet
- Forbes Et Al 2006Document17 pagesForbes Et Al 2006Usman AliNo ratings yet
- The Effects of Carbon Dioxide Removal On The Carbon CycleDocument16 pagesThe Effects of Carbon Dioxide Removal On The Carbon Cycleedgar steven mancipe casasNo ratings yet
- Geochem Geophys Geosyst - 2016 - Stranne - Dynamic Simulations of Potential Methane Release From East Siberian ContinentalDocument15 pagesGeochem Geophys Geosyst - 2016 - Stranne - Dynamic Simulations of Potential Methane Release From East Siberian ContinentaljyothsnasarikaNo ratings yet
- Carbon CycleDocument4 pagesCarbon Cyclecary19No ratings yet
- Geophysical Research Letters - 2011 - KrevorDocument5 pagesGeophysical Research Letters - 2011 - KrevormemeNo ratings yet
- International Journal of Greenhouse Gas Control: A B B ADocument15 pagesInternational Journal of Greenhouse Gas Control: A B B AOksita WidyayantiNo ratings yet
- Applied Clay Science: Aeslina Abdul Kadir, Abbas MohajeraniDocument8 pagesApplied Clay Science: Aeslina Abdul Kadir, Abbas MohajeraniEmmanuell KeithNo ratings yet
- Journal of Petroleum Science and Engineering: Constantin CranganuDocument5 pagesJournal of Petroleum Science and Engineering: Constantin Cranganuingjorge21No ratings yet
- Tao 2013Document8 pagesTao 2013zaarNo ratings yet
- An Experimental Investigation of The Roles of Water Content and Gas Decompression Rate For Outburst in Coal BriquettesDocument8 pagesAn Experimental Investigation of The Roles of Water Content and Gas Decompression Rate For Outburst in Coal BriquettesMJundiNo ratings yet
- Effect of Factors On The Hydrogen Composition in The Carburizing ProcessDocument7 pagesEffect of Factors On The Hydrogen Composition in The Carburizing ProcessAndi PrayogaNo ratings yet
- Calidad Del CementoDocument2 pagesCalidad Del CementoFranciscoCorreaJaraNo ratings yet
- Prosses Validation Protocol For EnrofolxacineDocument22 pagesProsses Validation Protocol For Enrofolxacineمحمد عطاNo ratings yet
- Waterstops PDFDocument26 pagesWaterstops PDFjmusopoleNo ratings yet
- Bourdon Gauge: Ithnaini Muhamed Kamil MRS 141104Document10 pagesBourdon Gauge: Ithnaini Muhamed Kamil MRS 141104Ithnaini KamilNo ratings yet
- History of Photo Volatic CellDocument12 pagesHistory of Photo Volatic CellKarthick ThiyaguNo ratings yet
- Organic Chemistry I - Simple Book PublishingDocument9 pagesOrganic Chemistry I - Simple Book PublishingMandyNo ratings yet
- Analise de VálvulaDocument10 pagesAnalise de VálvulaCristiano ScheuerNo ratings yet
- Ing. Emerson Escobedo: Universidad "Jose Carlos Mariategui" Escuela de Ingenieria CivilDocument20 pagesIng. Emerson Escobedo: Universidad "Jose Carlos Mariategui" Escuela de Ingenieria CivilChristian GutierrezNo ratings yet
- Gaskets&Seals ASMDocument3 pagesGaskets&Seals ASMAnonymous nw5AXJqjdNo ratings yet
- Removal of Methyl Orange Dye From Textile Effluent Using Adsorption On Chitosan Hydrogel BeadsDocument8 pagesRemoval of Methyl Orange Dye From Textile Effluent Using Adsorption On Chitosan Hydrogel BeadsESSENCE - International Journal for Environmental Rehabilitation and ConservaionNo ratings yet
- E3877 Optics FormulasDocument6 pagesE3877 Optics FormulasKaran DoshiNo ratings yet
- How To Measure Dissolved Oxygen in The BreweryDocument4 pagesHow To Measure Dissolved Oxygen in The BreweryRoo FaNo ratings yet
- Water Budget of Inter-Cropped Maize and Cassava On Bench TerracesDocument31 pagesWater Budget of Inter-Cropped Maize and Cassava On Bench TerracesSudharsananPRSNo ratings yet
- PolyolsDocument12 pagesPolyolsA MahmoodNo ratings yet
- Heats Sodium Hydroxide Solutions: SpecificDocument4 pagesHeats Sodium Hydroxide Solutions: SpecificTJ ArciagaNo ratings yet
- Refractories OverviewDocument54 pagesRefractories OverviewArun Kumar S.L.No ratings yet
- Mean Activity Coefficients of Electrolytes As A Function of ConcentrationDocument4 pagesMean Activity Coefficients of Electrolytes As A Function of ConcentrationLuis CarlosNo ratings yet
- Gerber Beams: Tip: Serious Errors Result From Lack of Unknown Forces at A Slit HingeDocument6 pagesGerber Beams: Tip: Serious Errors Result From Lack of Unknown Forces at A Slit HingeamiteshNo ratings yet
- Determination of Nickel As The Dimethylglyoxime Complex by SpectrophotometryDocument5 pagesDetermination of Nickel As The Dimethylglyoxime Complex by Spectrophotometryevenspase7859No ratings yet
- Stability Test ChamberDocument9 pagesStability Test Chambernishu_hainaNo ratings yet
- Macroetch Testing Steel Bars, Billets, Blooms, and Forgings: Standard Method ofDocument5 pagesMacroetch Testing Steel Bars, Billets, Blooms, and Forgings: Standard Method of陳勉中No ratings yet
- 06 05 16 Heko Bucket ElevatorDocument36 pages06 05 16 Heko Bucket ElevatorLeoncio Alexander Maza InfantesNo ratings yet
- EE Catalogue PDFDocument27 pagesEE Catalogue PDFssnNo ratings yet
- Work 4 - Perpandas-2019Document3 pagesWork 4 - Perpandas-2019hungNo ratings yet
- Combustion Chemstry1Document6 pagesCombustion Chemstry1Dr Mohammad AlzoubyNo ratings yet
- 24528Document3 pages24528reeta ramNo ratings yet
- New Piping Flexibility Rules in ASME B31.3 AppendixP - Becht & Diehl - ASME - 2006Document5 pagesNew Piping Flexibility Rules in ASME B31.3 AppendixP - Becht & Diehl - ASME - 2006Alvin SmithNo ratings yet
- Insert - NaOHD - SMS - SmpCln1+2 - SCCS.0005989914001c501.V26.enDocument9 pagesInsert - NaOHD - SMS - SmpCln1+2 - SCCS.0005989914001c501.V26.enARIF AHAMMED PNo ratings yet
- T7 - Mid-Semister Revisit and SterilizationDocument4 pagesT7 - Mid-Semister Revisit and SterilizationFishNo ratings yet