Professional Documents
Culture Documents
Marco Fuenzalida Et Al - Changes of The EPSP Waveform Regulate The Temporal Window For Spike-Timing-Dependent Plasticity
Uploaded by
FedrmOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Marco Fuenzalida Et Al - Changes of The EPSP Waveform Regulate The Temporal Window For Spike-Timing-Dependent Plasticity
Uploaded by
FedrmCopyright:
Available Formats
11940 The Journal of Neuroscience, October 31, 2007 27(44):11940 11948
Development/Plasticity/Repair
Changes of the EPSP Waveform Regulate the Temporal Window for Spike-Timing-Dependent Plasticity
Marco Fuenzalida, David Fernandez de Sevilla, and Washington Buno
Instituto Cajal, Consejo Superior de Investigaciones Cientficas, 28002 Madrid, Spain
Using spike-timing-dependent plasticity (STDP) protocols that consist of pairing an EPSP and a postsynaptic backpropagating action potential (BAP), we investigated the contribution of the changes in EPSP waveform induced by the slow Ca 2 -dependent K -mediated afterhyperpolarization (sAHP) in the regulation of long-term potentiation (LTP). The temporal window between Schaffer collateral EPSPs and BAPs in CA1 pyramidal neurons required to induce LTP was narrowed by a reduction of the amplitude and decay time constant of the EPSP, which could be reversed with cyclothiazide. The EPSP changes were caused by the increased conductance induced by activation of the sAHP. Therefore, the EPSP waveform and its regulation by the sAHP are central in determining the duration of the temporal window for STDP, thus providing a possible dynamic regulatory mechanism for the encoding of cognitive processes. Key words: temporal window; LTP regulation; increased conductance; EPSP profile; cyclothiazide; temporal coding
Introduction
Activity-dependent modifications of synaptic efficacy are thought to underlie learning and memory and to be essential for the maturation of the nervous system (Bliss and Collingridge, 1993; Malinow et al., 2000; Malinow and Malenka, 2002; Malenka and Bear, 2004; Dan and Poo, 2006). Different patterns of activity can increase or decrease synaptic efficacy, effects termed long-term potentiation (LTP) and long-term depression (LTD), respectively (Bliss and Lomo, 1973; Bliss and Collingridge, 1993; Malinow et al., 2000; Malinow and Malenka, 2002). At glutamatergic Schaffer collateral (SC) synapses of CA3 with CA1 hippocampal pyramidal neurons LTP is thought to originate postsynaptically and require the activation of the NMDA receptors (NMDARs) and a rise in spine Ca 2 caused by Ca 2 influx through NMDARs (Lynch et al., 1983; Malenka et al., 1989; Regehr and Tank, 1990). The induction of LTP classically involves presynaptic stimulation at high frequency, but lowfrequency stimulation paired with postsynaptic depolarization has also been commonly used (Bekkers and Stevens, 1990; Bliss and Collingridge, 1993; Malenka and Bear, 2004). An LTP induction protocol that appears to be closer to natural physiological conditions consists of the pairing of a presynaptic and a postsynaptic spike (Magee and Johnston, 1997; Markram et al., 1997; Bi and Poo, 1998). This LTP induction procedure, termed spikeReceived Feb. 28, 2007; revised June 20, 2007; accepted July 31, 2007. This work was supported by Direccion General de Investigacion Cientfica and Tecnologica, Ministerio de Ciencia and Tecnologa Grants BFI2002-01107 and BFU2005-07486, and Comunidad Autonoma de Madrid Grant GR/SAL/ 0877/2004 (W.B.). M.F. (on leave from Centro de Neurobiologa y Plasticidad del Desarrollo, Facultad de Ciencias, Universidad de Valparaso, Valparaso, Chile) is a doctoral fellow supported by a Ministerio de Relaciones Exteriores AGCI Grant. D.F.d.S. is a postdoctoral fellow supported by Comunidad Autonoma de Madrid Grant GR/SAL/0877/ 2004 and Ministerio de Ciencia and Tecnologa Grant BFU2005-07486. We thank Dr. A. Araque for his valuable suggestions on the final version of this manuscript. Correspondence should be addressed to Washington Buno, Instituto Cajal, Consejo Superior de Investigaciones Cientficas, Avenida Doctor Arce 37, 28002 Madrid, Spain. E-mail: wbuno@cajal.csic.es. DOI:10.1523/JNEUROSCI.0900-07.2007 Copyright 2007 Society for Neuroscience 0270-6474/07/2711940-09$15.00/0
timing-dependent plasticity (STDP) (Song et al., 2000), requires the generation of a subthreshold EPSP followed at short delays ( 0 30 ms) by a backpropagating action potential (BAP). This pairing protocol must be repeated to induce the sustained synaptic plasticity that characterizes LTP (Magee and Johnston, 1997; Markram et al., 1997; Bi and Poo, 1998; Abbott and Nelson, 2000; Feldman, 2000; Song et al., 2000; Sjostrom et al., 2001). The slow Ca 2 -dependent K -mediated afterhyperpolarization (sAHP) regulates synaptic efficacy (Sah and Bekkers, 1996; Borde et al., 1999; Le Ray et al., 2004) and the threshold for LTP induction (Sah and Bekkers, 1996; Le Ray et al., 2004). The sAHP also regulates the spread and integration of EPSPs and BAPs at apical dendrites of pyramidal neurons (Sah and Bekkers, 1996; Borde et al., 1999; Le Ray et al., 2004; Fernandez de Sevilla et al., 2007). Although these effects of the sAHP are expected to contribute to the regulation of STDP, their involvement is unknown. Using somatic whole-cell in vitro recordings in CA1 pyramidal cells, we analyzed the role of sAHP in the regulation of the temporal window during which the association of presynaptic and postsynaptic activity induces LTP with STDP protocols. We show that the temporal window is narrowed by the reduction of the amplitude and decay time constant of the EPSP caused by the increased membrane conductance ( gm) induced by the activation of the Ca 2 -dependent K channels that mediate the sAHP. Therefore, the sAHP effectively regulates the induction threshold and the magnitude of the LTP evoked by physiological stimulation protocols such as the STDP.
Materials and Methods
Procedures of animal care, surgery, and slice preparation were in accordance with the guidelines laid down by the European Communities Council. The procedures will be described briefly because they have been extensively detailed previously (Borde et al., 1995, 1999; Le Ray et al., 2004). Slice preparation. Young Wistar rats (14 16 d old) were decapitated, and the brain was removed and submerged in cold ( 4C) artificial CSF
Fuenzalida et al. The sIAHP Regulates the Induction of STDP
J. Neurosci., October 31, 2007 27(44):11940 11948 11941
(ACSF) (in mM): 124.00 NaCl, 2.69 KCl, 1.25 KH2PO4, 2.00 Mg2SO4, 26.00 NaHCO3, 2.00 CaCl2, and 10.00 glucose. The pH was stabilized at 7.4 by bubbling the ACSF with carbogen (95% O2, 5% CO2). Transverse hippocampal slices (300 350 m) were cut with a Vibratome (3000; Pelco, St. Louis, MO) and incubated in the ACSF ( 1 h at room temperature, 20 22C). Slices were transferred to a 2 ml chamber fixed to an upright microscope stage (BX51WI; Olympus, Tokyo, Japan) equipped with infrared differential interference contrast video microscopy and a 40 waterimmersion objective. Slices were superfused with carbogen-bubbled ACSF (2 ml/min) and maintained at room temperature. All recordings were made under picrotoxin (50 M), whereas 2-amino-5-phosphonopentanoic acid (APV) (50 M), nimodipine (10 M), thapsigargin, (1 M), 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288) (50 M), apamin (100 nM), isoproterenol (10 M), and cyclothiazide (CTZ) (100 M) were added to the ACSF as needed. Electrophysiology. Whole-cell recordings from soma of CA1 pyramidal cells were performed with patch pipettes (4 8 M ) filled with an internal solution that contained the following (in mM): 135 K-MeSO4, 10 HEPES, 0.5 EGTA, 2 Na2-ATP, and 0.4 Na3-GTP, buffered to pH 7.27.3 with KOH. In some experiments, the intracellular solutions contained heparin (5 mg/ml), ruthenium red (100 M), or BAPTA (50 mM). The BAPTA solution contained the following (in mM): 60 K-MeSO4, 10 HEPES, 50 BAPTA-K4, 2 Na2-ATP, and 0.4 Na3-GTP, buffered to pH 7.27.3 with KOH. Chemicals were purchased from Sigma-Aldrich Qumica (Madrid, Spain), Tocris (Biogen Cientfica, Madrid, Spain), and Alomone Labs (Jerusalem, Israel). Recordings were performed both in the current- and voltage-clamp modes using an Axoclamp-2B amplifier (Molecular Devices, Union City, CA). In voltage-clamp experiments, the holding potential (Vh) was adjusted to 65 mV. In current-clamp conditions, the membrane potential (Vm) was set to the same value by injecting DC current as needed, except when indicated otherwise. In the voltage-clamp configuration, the series resistance was compensated to 70%, and neurons were accepted only when the seal resistance was 1 G and the series resistance (714 M ) did not change 10% during the experiment. Current-clamp recordings were rejected if the resting Vm depolarized to values more than 50 mV. The liquid junction potential was measured ( 6 mV) but was not corrected. Voltage- and current-clamp data were high-pass filtered at 3.0 kHz and sampled at rates between 6.0 and 10.0 kHz, through a Digidata 1322A (Molecular Devices). The pClamp programs (Molecular Devices) were used to generate stimulus timing signals and transmembrane current pulses and to record and analyze data. The sAHPs were activated by a depolarizing current pulse (intensity, 0.5 nA; duration, 200 ms) applied through the somatic recording electrode. The depolarizing pulse induced a burst of spikes (7.4 2.3; n 41) that was followed by the Ca 2 activated K -mediated medium AHP (mAHP) and sAHP with faster and slower kinetics, respectively. EPSPs were evoked by stimulation of SCs with a bipolar extracellular electrode (diameter, 60 m; tip separation, 100 m) placed in the stratum radiatum 150 m from the recorded neuron. To allow comparisons between experiments, the amplitude of the EPSP was set to similar values (4 6 mV) 50% under the threshold that could evoke spikes. A Grass S88 and stimulus isolation unit (SIU; Grass Instruments, Quincy, MA) generated the simulation pulse protocols. In some experiments, a separate group of SC fibers was stimulated with a second electrode (as above) connected to a Cibertec SIU (Cibertec, Madrid, Spain) driven by the Clampex program. Pairedpulse response (PPR) tests between the two stimulation sites were used to determine that separate groups of fibers were being stimulated. Ca2 imaging. Simultaneous electrophysiology recordings and intracellular Ca 2 imaging were obtained. The latter was performed by fluorescence microscopy with the Ca 2 indicator fluo-3 (Invitrogen, Eugene, OR). Patch pipettes were filled with the internal solution containing 50 100 M Fluo-3. Imaging experiments were performed after a 10 15 min stabilization period that allowed the equilibration of the dye in the soma and apical dendritic shaft. Cells were illuminated every 200 ms at 490 nm during 40 ms with a monochromator (Polychrome IV; TILL Photonics, Planegg, Germany), and successive images were obtained at 5 s 1 with a cooled monochrome CCD camera (4920; Cohu, San Diego, CA) attached to the Olympus microscope, which was
equipped with a filter cube optimized for Fluo-3 (Chroma Technology, Rockingham, VT). Camera control, synchronization with electrophysiological measurements, and quantitative epifluorescence measurements were made with the Imaging Workbench software (INDEC BioSystems, Santa Clara, CA). Changes in fluorescence signals were expressed as the proportion (percentage) of relative change in fluorescence ( F/F0), where F0 is the prestimulus fluorescence level when the cell is at rest, and F is the change in fluorescence during activity. Plots of Ca 2 signal variations versus time were obtained off-line at specified regions of interest from stored image stacks and expressed as F/F0. Corrections were made for indicator bleaching during trials by subtracting the signal measured under the same conditions when the cell was not stimulated. A baseline control EPSC was evoked under voltage clamp by repeated stimulation at 0.3 Hz with the same intensity as EPSPs. The recording was then switched to the current-clamp mode, and a single EPSP was paired with a single postsynaptic action potential evoked by a transmembrane current pulse (3 ms, 0.52 nA) applied at delays of 2 40 ms through the somatic recording electrode. This LTP induction protocol was repeated 60 times at 1 s 1 (Bi and Poo, 1998). The magnitude of the LTP was measured under voltage clamp 2535 min after the end of induction protocol and expressed as a proportion (percentage) of the baseline control EPSC amplitude. The presynaptic or postsynaptic origin of the changes in EPSC amplitude was tested using the PPR test (Bekkers and Stevens, 1990; Fernandez de Sevilla et al., 2002). Data analysis. Data were analyzed with the pClamp programs (Molecular Devices). Statistical analysis was performed using Origin 7.0 (OriginLab, Northampton, MA). Results are given as mean SEM (n number of cells), and percentages are presented as percentage of control. Statistical analysis was performed using Students two-tailed t test, the difference was considered statistically significant at p 0.05, and p 0.01 and p 0.001 are also indicated.
Results
The sAHP raises the threshold for LTP induction The STDP protocol at an EPSPBAP delay of 10 ms induced a strong, long-lasting increase of EPSC peak amplitude or LTP (218.0 22.2%; p 0.05; n 20) (Fig. 1 A, top records, B, control, C, open circles). In contrast, no LTP was induced when the pre-postsynaptic pairing interval was increased to 30 ms (98.5 2.5%; p 0.05; n 10) (Fig. 2 B). However, reducing the pairing interval to 5 ms resulted in a larger LTP (see below), indicating that within a temporal window the ability of the STDP protocol to induce LTP decreases rapidly with the time delay between the EPSP and BAP (Bi and Poo, 1998; Abbott and Nelson, 2000; Song et al., 2000; Dan and Poo, 2006) (see below). The sAHP decreases excitability and may regulate the induction of LTP by the STDP by both (1) controlling action potential generation (Madison and Nicoll, 1984; Lancaster and Adams, 1986; Le Ray et al., 2004) and (2) reducing synaptic efficacy by means of modifying the amplitude, shape, and propagation of EPSPs at the apical dendrites of CA1 pyramidal cells (Sah and Bekkers, 1996; Fernandez de Sevilla et al., 2007). Therefore, we designed experiments to investigate whether the sAHP could control the induction threshold of the LTP evoked by STDP protocols. The sAHP was evoked by a depolarizing current pulse applied through the somatic electrode at a rate of 0.25 s 1 (see Materials and Methods), a repetition rate at which the sAHP is permanently activated (Borde at al., 1995) (Fig. 1 A, bottom records, B, with sAHP). In these conditions, the sAHP attained amplitudes between 5.9 and 9.8 mV (7.5 0.3 mV; n 10) from the resting Vm ( 58.8 0.8 mV; same cells). We first applied the STDP protocol at EPSPBAP delays of 10 ms. The 10 ms delay STDP protocol failed to induce LTP during the sAHP, and the control and postSTDP EPSCs were essentially identical ( p 0.5; n 12) (Fig. 1C, filled circles).
11942 J. Neurosci., October 31, 2007 27(44):11940 11948
Fuenzalida et al. The sIAHP Regulates the Induction of STDP
The sAHP narrows the critical temporal window for LTP induction The above results indicate that sAHP may prevent the induction of LTP with an EPSPBAP delay of 10 ms, but the ability of the STDP protocol to induce LTP increases with the reduction of the EPSP BAP delay (Bi and Poo, 1998; Song et al., 2000; Dan and Poo, 2006), suggesting that the sAHP may not inhibit LTPs induced with briefer EPSPBAP delays. The sAHP reduces the amplitude and of EPSPs at CA1 pyramidal neuron dendrites to 77.0 0.5% and d 50.8 11.7%, respectively (n 8; p 0.05 in both cases) (Fig. 2C) (Sah and Bekkers, 1996; Fernandez de Sevilla et al., 2007), and the briefer EPSPs could effectively narrow the temporal window of coincidence between EPSP and BAP required to induce the LTP. We examined the changes in the waveform of the EPSP and the BAP before and during sAHP. During the sAHP, the EPSP amplitude decreased (from 3.4 0.2 to 2.4 0.2 mV, a 29.7 5.4% reduction; p 0.05; n 10), and the EPSP decayed with a faster than in the control (from 62.1 3.8 ms to 33.2 2.4 ms, a 53.2 3.1% reduction; p 0.01; n 10) (Fig. 2C). In contrast, neither the amplitude nor the waveform of the BAP were affected by the sAHP, and this was true both for somatic and dendritic recordings at the sites where the LTP was induced 150 M from the soma ( p 0.05; n 10) (Fig. 2 D). Therefore, we tested whether the LTP could be induced during the sAHP when the time interval between the EPSP and BAP was reduced below 10 ms. Although with an EPSPBAP interval of 10 ms the sAHP suppressed the LTP ( p 0.05; n 6) (Fig. 2 A), a second STDP protocol at a briefer delay of 5 ms delivered in the same cells 10 min later was able to induce the LTP during the sAHP (160.8 9.7%; p 0.05; n 6), suggesting that the sAHP acted by narrowing the temporal window for coincidence detection required to induce the LTP with STDP protocols (Fig. 2 A). Figure 1. The sAHP suppresses the induction of LTP. A, Left, Diagram showing recording and stimulating electrode locations. To quantify the modifications of the Top and bottom right, Representative current-clamp records showing the STDP protocol (10 ms) delivered in the control and temporal window induced by the sAHP, during the sAHP. The sAHP was activated continuously by repeated depolarizing pulses (0.5 nA, 200 ms, at 0.25 s 1) that we performed experiments with STDP persistently hyperpolarized the cell during the STDP protocol. Insets, Expanded versions of the pairing between EPSP and BAP at protocols at different EPSPBAP delays in a 10 ms delay in the control and during sAHP. Stim., Stimulation. B, Averaged EPSCs (n 10 as in other cases) recorded before (1) control conditions (i.e., in the absence of and 30 min after (2) the STDP protocol delivered in control condition (top records) and during the sAHP (bottom records). C, Time sAHP activation). The LTP was consis- course of the changes in EPSC amplitude induced by the 10 ms pairing interval STDP protocol (open arrow, as in all other figures) in the control ( sAHP, open circles) and during the sAHP ( sAHP, filled circles). tently generated (166.6 10.2%; p 0.05; n 6) at delays of 15 ms, and the LTP 0.05; n 10) or briefer (at 2 ms, the LTP was 201.6 15.0%; p increased in magnitude (231.6 5.7%; p 0.001; n 6) when 0.05; n 6). With EPSPBAP delays of 10 ms or longer, the sAHP the EPSPBAP interval was reduced to 5 ms. With EPSPBAP suppressed the LTP ( p 0.05; n 6) (Fig. 2 B). Therefore, the intervals of 30 ms and above, the LTP was not induced (Fig. 2 B). sAHP reduced the magnitude of the LTP and narrowed the temIn contrast, during the sAHP, the STDP protocols induced the LTP only if the EPSPBAP interval was 5 ms (129.1 4.1%; p poral window of coincidence. We plotted the functional relation-
Fuenzalida et al. The sIAHP Regulates the Induction of STDP
J. Neurosci., October 31, 2007 27(44):11940 11948 11943
cations by measuring the changes in the amplitude of the voltage responses evoked by depolarizing current pulses without and with sAHPs of different magnitudes from different experiments (n 30) (Fig. 3 A, B). We measured the changes in EPSP amplitude and decay as a function of the gm raise and found that both functional relationships were well described by linear functions with negative slopes, indicating an inverse relationship of both amplitude and decay of the EPSPs with the increased gm (Fig. 3B), suggesting that the change in gm was the central factor underlying the effects of the sAHP. We also measured the relationship between the gm change caused by the activation of the sAHP and the magnitude (percentage) of the LTP. There was an inverse relationship of the gm with the magnitude of the LTP evoked by pairing at EPSPBAP delays of 10 ms, indicating that the LTP was gradually smaller with larger gm values (Fig. 3C). In addition, when the rise in gm induced by the sAHP activation was 140% of the control value, the LTP was suppressed ( p 0.05; n 30), indicating a threshold value of the gm over which the LTP was prevented. Figure 2. The sAHP narrows the temporal window for LTP. A, The LTP is suppressed by the sAHP induced with a 10 ms Together, these results suggest that the EPSPBAP interval. In contrast, a 5 ms interval induced LTP during the sAHP in the same cells (n 6). The insets show averaged EPSC recorded before (1) and 5 min after (2) the first STDP (10 ms) protocol and 20 min after the second STDP (5 ms) protocol (3). increased gm caused by the activation of B, Summary data showing the functional relationship between the EPSC amplitude (%) and the EPSPBAP interval in control the sAHP regulated both the induction 9.3 0.5 ms and R 2 threshold and the magnitude of the LTP. conditions (open circles) and during the sAHP (filled circles). The control single exponential fit showed 2 0.97 (n 36), and during the sAHP, 2.5 0.5 ms and R 0.99 (n 32). *p 0.05; **p 0.01. C, Representative The results also suggest that the gm reduces current-clamp records showing averaged EPSPs recorded before and during the sAHP (gray bars). The inset shows superimposed the temporal window of coincidence beEPSPs recorded before (black trace) and during (gray trace) the sAHP. D, Left, Diagram showing somatic and dendritic (150 m) tween the EPSP and BAP required to inrecording electrode locations. Right, Representative somatic and dendritic recordings showing EPSPs and APs before and during duce the LTP by decreasing the amplitude the sAHP (black and gray traces, respectively). and decay of the EPSP. Therefore, the properties of the EPSP are a key factor regships between the EPSPBAP interval and the magnitude of the ulating the temporal window of coincidence. LTP (% EPSC amplitude), which were well fitted by single exponential functions both in the control condition and with the Hyperpolarization and reblock of NMDARs do not contribute sAHP (Fig. 2 B). Exponential fits were significantly different ( p to the effects of the sAHP 2 9.3 0.5 ms and a R 0.05) with 0.97 in the control (n 2 Membrane hyperpolarization during the sAHP and Mg 2 re2.5 0.5 ms and a R 36) and 0.99 (n 32) during the block of NMDARs could effectively contribute to the regulation sAHP, respectively, confirming that the sAHP effectively narof the LTP by the sAHP by diminishing the influx of Ca 2 . To rowed the temporal window of coincidence required to induce distinguish between those possibilities, we performed two differthe LTP. ent tests. First, we delivered the 10 ms EPSPBAP delay protocol during a simulated sAHP that was generated by applying a LTP induction is regulated by the raise in gm underlying current waveform obtained from an averaged real sAHP the sAHP through the somatic electrode (Fig. 4 A). The STDP protocol deWe have previously shown that the modifications in EPSP amplilivered during the simulated sAHP induced a robust LTP tude and duration were induced by the gm raise that parallels the (185.2 7.2%; p 0.05; n 4), indicating that hyperpolarizaactivation of the sAHP (Fernandez de Sevilla et al., 2007). Contion caused by the simulated sAHP did not affect the LTP (Fig. sequently, we tested whether a relationship existed between the 4 A, B). We also applied the STDP protocol during a real sAHP EPSP properties and the changes in gm. The shunting of the generated with depolarizing pulses while holding the cell at 85 EPSP by the increased gm may be caused by the AP itself (Hausser mV (close to the K reversal potential) by continuous injection et al., 2001) rather than by the rise in gm that parallels the sAHP of hyperpolarizing current (Fig. 4C,D). In this condition the activation. However, pairing the EPSP with the BAP at 10 ms in sAHP effectively suppressed the LTP (104.6 10.6%; p 0.05; the control condition revealed that the peak amplitude of the n 6), although the hyperpolarization associated with the sAHP EPSP was unaffected by the AP ( p 0.05; n 8), suggesting that activation was absent (Fig. 4C,D). However, when the STDP prothe rise in gm associated with the activation of the sAHP was the key factor in modifying the EPSP. We estimated the gm modifitocol was delivered at the same hyperpolarized Vm of 85 mV in
11944 J. Neurosci., October 31, 2007 27(44):11940 11948
Fuenzalida et al. The sIAHP Regulates the Induction of STDP
sential, but in contrast, the gm change underlying the sAHP is vital in terms of blocking the LTP induction by the STDP protocol (Fig. 4 D). To confirm that the increased gm during the sAHP was the key factor in the suppression of the LTP, we applied brief test depolarizing current pulses (10 pA, 200 ms) during the simulated and real sAHP. The amplitude and time constant ( ) of the membrane depolarization evoked by the brief test pulses were essentially identical before and during the simulated sAHP ( p 0.05 in both cases; n 6), consistent with the absence of modifications in gm caused by the possible activation of the hyperpolarizationactivated cationic H-current (Ih) or by reblock of NMDARs by extracellular Mg 2 . In addition, the gm increments associated with the real sAHP were similar at both resting and hyperpolarized Vms (at 60 mV, 161.2 11.1% and 85 mV, 154.5 8.3%, respectively; p 0.05 in both cases; n 6), although membrane hyperpolarization was present at 60 mV and absent at 85 mV, respectively (data not shown). The above results are consistent with the suppression of the LTP being caused by the increase in gm induced by the opening of the Ca 2 -dependent K channels that mediate the sAHP. In contrast, the results also imply that in the present conditions, membrane hyperpolarization per se had no effect in the induction threshold of LTP. Blockade of the sAHP prevented the suppression of the LTP When the sAHP was inhibited (by 80.2 10.2%; p 0.05; n 8) by perfusion with the -adrenergic receptors agonist isoproterenol (10 M) (Fig. 5A), the STDP protocol (10 ms) induced a robust LTP (Fig. 5 B, D). Isoprotorenol blocked the sAHP without modifying the spike burst induced by the depolarizing pulse (data not shown), suggesting that the absence of a contribution of the initial spike response in the suppression of the LTP. Although activation of -adrenergic receptors can enhance the homosynaptic (Yang et al., 2002) and associative LTP (Lin et al., 2003), we could not detect changes in the magnitude of the LTP ( p 0.05; n 8) nor modification of the control EPSC under isoproterenol ( p 0.05; n 4). In addition, blockade of the sAHP with isoproterenol did not significantly modify the intracellular Ca 2 signal induced by the depolarizing pulse used to trigger the sAHP (in control, the Ca 2 signal was 15.8 1.4% and with isoproterenol, 14.5 1.2%; n 5; p 0.05) (Fig. 5A), suggesting that the intracellular Ca 2 raise was not involved in the suppression of the LTP. The above results are consistent with the notion that the sAHP specifically modifies the induction threshold and the temporal window of the LTP. However, we could not record the Ca 2 signal in the spines, which would have provided conclusive information on the interactions between the depolarization provided by the modified EPSP and the BAP in control solution and with isoproterenol. The mAHP is also activated by the pulse protocol and may contribute to the described effects. We tested the blockade of the mAHP with apamin (100 nM), which did not prevent the sAHP, to suppress the LTP (Fig. 5C,D). In addition, Ih could be activated by the hyperpolarization induced by the sAHP and modify the induction threshold of the LTP by the STDP protocol. The specific blocker of Ih channels, ZD7288 (50 M), did not modify the inhibition of the STDP induced by activation of the sAHP (Fig. 5D). The above results are consistent with the lack of contribution of both the mAHP and Ih to the blockade of the LTP and suggest that the suppression of the LTP is exclusively mediated by the sAHP.
Figure 3. LTP induction is regulated by the rise in gm underlying the sAHP. A, Representative current-clamp records showing the changes of gm during the sAHP estimated from responses evoked by brief depolarizing current pulses (10 pA, 200 ms). The inset shows superimposed responses before (black trace) and during (gray trace) the sAHP. B, Summary data showing the relationship between the gm (%) and the amplitude (filled circles) and decay (open circles) of the EPSP during the sAHP (n 30). C, Summary data illustrating the relationship between gm and magnitude of the LTP (%). Note that when the gm increased 140%, the LTP was suppressed (same cells).
the absence of sAHP activation, a robust LTP was induced (201.1 22.6%; p 0.05; n 4). The above results are consistent with a scenario in which the hyperpolarization associated with the sAHP activation is not es-
Fuenzalida et al. The sIAHP Regulates the Induction of STDP
J. Neurosci., October 31, 2007 27(44):11940 11948 11945
(Fig. 2 B) and 9.3 1.3 ms with the sAHP under CTZ were essentially identical ( p 0.05) (Fig. 6C). We also compared the amplitude and decay of EPSPs in control conditions and during the sAHP both under 100 M CTZ and in control ACSF. CTZ increased the amplitude and decay of EPSPs relative to the corresponding controls in the absence of sAHP activation (by 145.7 0.5% and 159.4 7.7%, respectively; p 0.05 in both cases; n 10) (Fig. 6C). The EPSP amplitude and were also increased by CTZ during the sAHP (59.0 2.4% and 118.1 9.5%, respectively, in both cases; p 0.05; n 10) (Fig. 6C). In addition, CTZ did not modify BAPs ( p 0.05; n 10), the input resistance ( p 0.05; same cells), nor the sAHP ( p 0.05; n 10). The changes induced by CTZ on the EPSC waveform clearly illustrate that the EPSP modification was caused by the inhibition of AMPA receptor desensitization (Fig. 6 D). Therefore, CTZ can effectively widen Figure 4. Hyperpolarization and reblock of NMDARs do not contribute to the effects of the sAHP. A, Records showing the STDP protocol (10 ms) delivered during the simulated sAHP. B, Summary data showing that the sustained hyperpolarization during the the temporal window both in the absence simulated sAHP failed to suppress the LTP. The insets are averaged EPSCs recorded before (1) and 30 min after (2) the STDP of sAHP activation and with the sAHP, in protocol. C, STDP protocol (10 ms) delivered during the sAHP while holding the Vm at 85 mV by continuous current injection. D, the latter case counteracting the effects of Summary data illustrating the suppression of the LTP by the sAHP when the STDP protocol (10 ms) was delivered at 85 mV (filled the sAHP on the induction of the LTP. The circles). In the absence of sAHP, the same STDP protocol delivered at 85 mV induced a robust LTP. The insets show EPSC averages above results are consistent with the norecorded before (1) and 30 min after (2) the STDP protocol with the sAHP. Stim., Stimulation. tion that the amplitude and waveform of the EPSPs are key factors controlling the duration of the temporal window for The amplitude and waveform of the EPSP regulates the STDP both in control conditions and during the sAHP. temporal window The LTP in the CA1 region is input specific (Bliss and ColThe above results suggest that the sAHP narrows the temporal lingridge, 1993; Malinow and Malenka, 2002; Malenka and Bear, window by reducing the amplitude and decay of the EPSP. 2004; Dan and Poo, 2006). However, it is not known whether the Therefore, manipulations that increase EPSP amplitude and dusuppression of the LTP by the sAHP is restricted to particular ration should broaden the temporal window both in control conregions or is widespread. We tested the input specificity of the ditions and during the sAHP, thus possibly counteracting the sAHP by stimulating different groups of SC fibers in the apical effects of the sAHP on the LTP. We tested the effects of CTZ (100 dendrite close to and far from the soma (proximal, 150 m and M), which inhibits AMPA receptor desensitization and indistal, 250 m, respectively). The absence of common fibers in creases the amplitude and duration of EPSPs (Arai et al., 1994; proximal and distal inputs was confirmed by the lack of crossPelletier and Hablitz, 1994) (Fig. 6C). facilitation. The LTP was induced at the input that received the In the absence of sAHP activation, the functional relationship STDP protocol at a 10 ms pairing interval (proximal, 215.1 between the EPSPBAP intervals and the magnitude of the LTP 14.9% and distal, 147.7 8.9%; p 0.05; n 8) but not the other (% EPSC amplitude) revealed that under the CTZ, the temporal input that was used as a control ( p 0.05; same cells). The STDP window was widened to 30 ms, and larger LTPs (than control) protocol delivered during the sAHP failed to induce LTP in both were induced by briefer EPSPBAP intervals (Fig. 6 B). In the inputs ( p 0.05; same cells). presence of sAHP activation, robust LTPs (larger than control) The LTP induced by high-frequency stimulation of SC synwere generated under 100 M CTZ with a 10 ms pairing interval apses is thought to be mediated postsynaptically by Ca 2 influx (191.3 5.1%; p 0.05; n 6) (Fig. 6 A, B). Larger LTPs were through NMDARs (Lynch et al., 1983; Malenka et al., 1989; Realso induced by pairing intervals between 2 and 20 ms during gehr and Tank, 1990; Bliss and Collingridge, 1993). We tested the sAHP (Fig. 6 B), suggesting that CTZ counteracted the effects of effects of blockade of NMDARs with APV (50 M; n 8) and of the sAHP (Fig. 2 B). Under CTZ, the functional relationships chelation of intracellular Ca 2 with BAPTA (50 mM; n 4), between the EPSPBAP pairing intervals and the LTP (% EPSC which inhibited the LTP induced by STDP protocols. We also amplitude) were well fitted by single exponential functions (Fig. examined the effect of nimodipine (10 M), which selectively 6 B). The fit had a of 13.7 2.1 ms (R 2 0.99) with the sAHP blocks L-type Ca 2 channels that play a major role in and a of 9.3 1.3 ms (R 2 0.99) in the control, respectively depolarization-mediated Ca 2 influx in CA1 pyramidal cells ( p 0.05; n 34 and n 30, respectively), confirming that the (Raymond and Redman, 2002, 2006; Zucker, 2002) and could sAHP effectively narrowed the temporal window of coincidence contribute to the LTP (Yasuda et al., 2003; Moosmang et al., required to induce the LTP. Therefore, CTZ counteracted the 2005). However, nimodipine (10 M) did not modify the LTP 9.3 0.6 ms effects of the sAHP on LTP because the control induced by the STDP protocol ( p 0.05; n 10). Endoplasmic
11946 J. Neurosci., October 31, 2007 27(44):11940 11948
Fuenzalida et al. The sIAHP Regulates the Induction of STDP
reticulum (ER) internal stores are another potential source of Ca 2 that could contribute to the LTP (Schiegg et al., 1995; Raymond and Redman, 2002, 2006). However, the IP3 receptor antagonist heparin (5 mg/ml) and the ryanodine receptor antagonist ruthenium red (100 M) in the pipette solution, as well as depletion of internal Ca 2 stores with thapsigargin (1 M), respectively, were unable to prevent the induction of LTP. We analyzed the modifications of paired-pulse responses to test for presynaptic changes in release probability paralleling the LTP. All of the EPSCs tested showed paired-pulse facilitation that did not change with the induction of the LTP (p 0.05; n 10), consistent with the LTP induced by STDP protocols being exclusively mediated by postsynaptic mechanisms.
Discussion
We present original evidence indicating that activation of the sAHP narrows the temporal window between EPSPs and BAP Figure 5. The LTP was induced after blockade of the sAHP. A, Top left, Superfusion with 10 M isoproterenol markedly reduced required to induce the LTP with STDP the sAHP (n 8; p 0.01). Bottom left, Representative records of the intracellular Ca 2 signal recorded at the soma during the protocols. Our results suggest that changes sAHP and under inhibition of sAHP with 10 M Isoproterenol (black and gray traces, respectively). Right, Images showing changes in the amplitude and decay kinetics of SC in intracellular Ca 2 signals before (1), during the sAHP (2), and after (3) the sAHP was inhibited with 10 M isoproterenol. B, EPSPs, caused by the rise in gm associated Pooled data showing that under blockade of the sAHP with 10 M isoproterenol (Iso), the 10 ms STDP protocol induced a robust with the opening of the K channels that LTP (n 8). Top, Insets show averaged EPSCs recorded before (1) and 30 min after (2) STDP protocol. C, Inhibition of the mAHP mediate the sAHP (Sah and Bekkers, 1996; with apamin (100 nM) did not alter the ability of the sAHP to suppress the LTP (filled circles; n 6) and had no effect on the LTP Fernandez de Sevilla et al., 2007), are the induced in the absence of sAHP activation (open circles; n 6). D, Summary data showing the effects of Iso (10 M) and apamin key factors in determining the width of the (Apa; 100 nM), as well as the inhibition of Ih with ZD7288 (50 M), on the LTP (% EPSC amplitude) during the sAHP. P-AHP, Pairing during AHP. ***p 0.001. The numbers in the bars represent sample size (n). temporal window in STDP. The timing requirement for induction tance)] in pyramidal neuron dendrites may modify STDP of associative LTP with STDP protocols has been described both (Shouval et al., 2002; Watanabe et al., 2002; Dan and Poo, 2006), for hippocampal (Magee and Johnston, 1997; Bi and Poo, 1998) and the EPSP-dependent inactivation of transient IA in the denand neocortical pyramidal neuron synapses (Markram et al., drites contributes to the induction of LTP by STDP protocols in 1997; Feldman, 2000). The induction of LTP requires an EPSP CA1 pyramidal cells by boosting BAPs (Watanabe et al., 2002). In followed by a BAP at delays under 10 20 ms (Magee and addition, activation of -adrenergic receptors (Foehring et al., Johnston, 1997; Markram et al., 1997; Bi and Poo, 1998; Feldman, 1989; Fernandez de Sevilla et al., 2007), muscarinic receptors 2000; Song et al., 2000; Sjostrom et al., 2001). The magnitude of (Huerta and Lisman, 1993; Segal and Auerbach; 1997), or LTP depends on the EPSPBAP interval, with briefer intervals metabotropic glutamate receptors (Melyan et al., 2002) and horinducing larger LTPs (Bi and Poo, 1998; Song et al., 2000; Sjos mones (Carrer et al., 2003), which exert a robust, although untrom et al., 2001; Dan and Poo, 2006). In agreement with previ specific inhibition of the sAHP, can regulate the threshold for ous reports in hippocampal SCCA1 synapses in slices (Lin et al., LTP induction in CA1 pyramidal neurons. Indeed, neurotrans2003), we show that the temporal window for the induction of mitters that reduce the amplitude of the sAHP, and therefore LTP is 20 ms and that reductions of the EPSPBAP interval increase dendritic excitability, facilitate the spread of EPSPs and increase the LTP. favor BAPs, crucial factors required to reach a critical dendritic The pre-postsynaptic timing of activity, which determines the depolarization and Ca 2 elevation for LTP induction (Kampa et temporal window, depends mainly on the dendritic morphology al., 2004, 2006; Le Ray et al., 2004). and the interaction of dendritic ionic channels with EPSPs and We propose that the reduced amplitude and duration of the BAPs. The activation of dendritic ionic conductances may proEPSP induced by the sAHP narrows the temporal window of foundly change the temporal interaction between EPSPs and coincidence between the EPSP and BAP by decreasing the spine BAPs in pyramidal cells and may modulate the induction of LTP depolarization required to activate NMDARs and induce the by dynamically regulating the dendritic spread and waveform of LTP. Consistent with this view, we show that the sAHP reduces EPSPs and BAPs (Migliore et al., 1999; Shouval et al., 2002; Wathe amplitude and duration of EPSPs and that the effects of the tanabe et al., 2002). These effects on EPSPs and BAPs may modify sAHP are counteracted by increasing the amplitude and duration the temporal window for LTP (Bi and Poo, 1998; Song et al., of the EPSP via inhibiting AMPA receptor desensitization with 2000; Sjostrom et al., 2001; Dan and Poo, 2006). Indeed, the CTZ. Indeed, CTZ effectively widens the temporal window for density of dendritic potassium conductance [i.e., IA and Ca 2 LTP induction not only when the sAHP is activated but also in dependent K channels (large conductance and small conduc-
Fuenzalida et al. The sIAHP Regulates the Induction of STDP
J. Neurosci., October 31, 2007 27(44):11940 11948 11947
Ca 2 signals in the spine produced by the interaction of the EPSP and the BAP (Koester and Sakmann, 1998; Schiller et al., 1998; Nevian and Sakmann, 2006). Other mechanisms that increase Ca 2 concentration, such as activation of voltage-gated Ca 2 channels or release from intracellular Ca 2 ER stores either by the mechanism of Ca 2 -induced Ca 2 release via activation of Ca 2 -sensitive ryanodine receptors or by the production of IP3 and activation of IP3 receptors, can increase Ca 2 in spine and dendrite and could contribute to the LTP (Schiegg et al., 1995; Raymond and Redman, 2002, 2006). However, blockade of L-type Ca 2 channels with nimodipine, inhibition of ryanodine, and IP3 receptors with ruthenium red and heparin in the pipette solution, respectively, did not modify the LTP (present results). Therefore, in our experimental conditions, the LTP induced by Figure 6. The EPSP waveform regulates the temporal windows of STDP/LTP. A, Under superfusion with 100 M CTZ, the STDP the STDP protocols is entirely indepenprotocol (10 ms) delivered during the sAHP induced a robust LTP. The inset shows EPSC averages before (1) and 30 min after (2) the 2 STDP protocol. B, Summary data illustrating the functional relationship between the EPSC amplitude (%) and the EPSPBAP dent both on Ca2 influx through L-type pairing interval under CTZ (100 M) (open circles), during the sAHP in control ACSF (gray circles), and with 100 M CTZ (filled channels and Ca release from ER stores. In conclusion, the activation of the 2.5 0.4 ms and R 2 0.99 (n 32); with the circles). The exponential fits show as follows: in control ACSF with the sAHP, 2 2 sAHP under CTZ, 9.3 1.3 ms and R 0.99; and in control ACSF with CTZ, 13.7 2.1 ms and R 2 0.99. Note that Ca -dependent K channels that mediCTZ broadened the temporal window both in the control and with the sAHP. *p 0.05. C, Representative EPSP averages in control ate the sAHP may act as a dynamic cellular ACSF and under CTZ (black and gray traces, respectively) before and during the sAHP. D, Left, Representative voltage-clamp switch that modifies the narrow critical inrecordings showing EPSCs in control conditions and with CTZ (black and gray traces, respectively). D, Right, Same as left but scaled terval between presynaptic and postsynapto the peak amplitude. tic activity necessary for the induction of LTP. Changes in the temporal window control conditions. The contrasting effects of the sAHP and CTZ may also function as a gain control mechanism that regulates the on the temporal window provide novel evidence consistent with magnitude of the LTP. Our results also point to a crucial funca key importance of the EPSP profile in STDP and specially in tional role of the amplitude and duration of the EPSP in controlestablishing the timing requirement for induction of associative ling the temporal window of the STDP, thus providing yet anLTP. Recent results support the above view because both in the other dynamic regulatory mechanism that may be decisive for the cortex (Feldman, 2000; Sjostrom et al., 2001) and hippocampal encoding of cognitive processes. It has been proposed that the cultures (Bi and Poo, 1998), the induction of LTP demands large sAHP level might determine the degree of synaptic plasticity and EPSPs suggesting that a critical depolarization must be provided learning (Disterhoft and Oh, 2006), and this function of the by the EPSP for the generation of the LTP by the STDP protocol. sAHP could be operative in STDP. Indeed, the EPSP waveform The contribution of the EPSP properties to STDP should be anmodifications that parallel the sAHP activation may function in alyzed in detail especially because it has been reported that a physiological conditions when action potential bursts are inpositive regulation of AMPA receptors may facilitate synaptic duced in CA1 pyramidal neurons by excitatory synaptic inputs as plasticity and learning (Staubli et al., 1994; Arai et al., 2004). We may occur during the theta rhythm in vivo (Nunez et al., 1987; show that the BAP was not modified by the sAHP in the soma and Kamondi et al., 1998) and under activation of NMDARs in vitro the apical dendrite at sites where the LTP was induced. These in which the sAHP plays a key role (Bonansco and Buno, 2003). results are consistent with an insignificant contribution of the modifications induced by the sAHP on the BAP. References Although the cellular mechanisms that mediate the LTP inAbbott LF, Nelson SB (2000) Synaptic plasticity: taming the beast. Nat Neurosci 3:1178 1183. duction by the STDP are not well understood, it has been estabArai A, Kessler M, Xiao P, Ambros-Ingerson J, Rogers G, Lynch G (1994) A lished that it relies on the activation of NMDARs and is mediated centrally active drug that modulates AMPA receptor gated currents. Brain by Ca 2 influx through NMDARs after the relief of the Mg 2 Res 638:343346. block caused by the combined depolarization induced by the Arai AC, Xia YF, Suzuki E (2004) Modulation of AMPA receptor kinetics EPSP and the BAP (Bi and Poo, 1998; Kampa et al., 2004, 2006; differentially influences synaptic plasticity in the hippocampus. NeuroFroemke et al., 2005). Blockade of NMDARs with APV and inscience 123:10111024. Bekkers JM, Stevens CF (1990) Presynaptic mechanism for long-term potracellular Ca 2 chelation with BAPTA loading suppressed the tentiation in the hippocampus. Nature 346:724 729. LTP, confirming that activation of NMDARs and the resulting Bi GQ, Poo MM (1998) Synaptic modifications in cultured hippocampal intracellular Ca 2 elevation are both necessary to induce the LTP neurons: dependence on spike timing, synaptic strength, and postsynap(present results). In addition, experimental evidence indicates tic cell type. J Neurosci 18:10464 10472. that the cooperative interplay between the activation of the EPSP Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term and the BAP boosts Ca 2 influx and that the temporal window of potentiation in the hippocampus. Nature 361:3139. STDP is dependent on the supralinear summation of voltage and Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission
11948 J. Neurosci., October 31, 2007 27(44):11940 11948 in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 232:331356. Bonansco C, Buno W (2003) Cellular mechanisms underlying the rhythmic bursts induced by NMDA microiontophoresis at the apical dendrites of CA1 pyramidal neurons. Hippocampus 13:150 153. Borde M, Cazalets JR, Buno W (1995) Activity-dependent response depres sion in rat hippocampal CA1 pyramidal neurons in vitro. J Neurophysiol 74:1714 1729. Borde M, Bonansco C, Buno W (1999) The activity-dependent potentiation of the slow Ca2 -activated K current regulates synaptic efficacy in rat CA1 pyramidal neurons. Pflugers Arch 437:261266. Carrer HF, Araque A, Buno W (2003) Estradiol regulates the slow Ca 2 activated K current in hippocampal pyramidal neurons. J Neurosci 23:6338 6344. Dan Y, Poo MM (2006) Spike timing-dependent plasticity: from synapse to perception. Physiol Rev 86:10331048. Disterhoft JF, Oh MM (2006) Learning, aging and intrinsic neuronal plasticity [review]. Trends Neurosci 29:587589. Feldman DE (2000) Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron 27:4556. Fernandez de Sevilla D, Cabezas C, de Prada AN, Sanchez-Jimenez A, Buno W (2002) Selective muscarinic regulation of functional glutamatergic Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol (Lond) 545:51 63. Fernandez de Sevilla D, Fuenzalida Marco, Porto Pazos Ana, Buno W (2007) Selective shunting of the NMDA EPSP component by the slow after hyperpolarization in rat CA1 pyramidal neurons. J Neurophysiol 97:32423255. Foehring RC, Schwindt PC, Crill WE (1989) Norepinephrine selectively reduces slow Ca 2 - and Na -mediated K currents in cat neocortical neurons. J Neurophysiol 61:245256. Froemke RC, Poo MM, Dan Y (2005) Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature 434:221225. Hausser M, Major G, Stuart GJ (2001) Differential shunting of EPSPs by action potentials. Science 291:138 141. Huerta PT, Lisman JE (1993) Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 364:723725. Kamondi A, Acsady L, Wang X-I, Buzsaki G (1998) Theta oscillations in somata and dendrites of hippocampal pyramidal cell in vivo: activity dependent phase precession of action potentials. Hippocampus 8:244 251. Kampa BM, Clements J, Jonas P, Stuart G (2004) Kinetics of Mg 2 unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J Physiol (Lond) 556:337345. Kampa BM, Letzkus JJ, Stuart GJ (2006) Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity. J Physiol (Lond) 574:283290. Koester HJ, Sakmann B (1998) Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc Natl Acad Sci USA 95:9596 9601. Lancaster B, Adams PR (1986) Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol 55:1268 1282. Le Ray D, Fernandez de Sevilla D, Porto AB, Fuenzalida M, Buno W (2004) Heterosynaptic metaplastic regulation of synaptic efficacy in CA1 pyramidal neurons of rat hippocampus. Hippocampus 14:10111025. Lin YW, Min MY, Chiu TH, Yang HW (2003) Enhancement of associative long-term potentiation by activation of beta-adrenergic receptors at CA1 synapses in rat hippocampal slices. J Neurosci 23:4173 4181. Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F (1983) Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature 305:719 721. Madison DV, Nicoll RA (1984) Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol (Lond) 354:319 331. Magee JC, Johnston D (1997) A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275:209 213. Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44:521.
Fuenzalida et al. The sIAHP Regulates the Induction of STDP Malenka RC, Kauer JA, Perkel DJ, Nicoll RA (1989) The impact of postsynaptic calcium on synaptic transmissionits role in long-term potentiation. Trends Neurosci 12:444 450. Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity Annu Rev Neurosci 25:103126. Malinow R, Mainen ZF, Hayashi Y (2000) LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol 10:352357. Markram H, Lubke J, Frotscher M, Sakmann B (1997) Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275:213215. Melyan Z, Wheal HV, Lancaster B (2002) Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34:107114. Migliore M, Hoffman DA, Magee JC, Johnston D (1999) Role of an A-type K conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J Comput Neurosci 7:515. Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T (2005) Role of hippocampal Cav1.2 Ca 2 channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25:98839892. Nevian T, Sakmann B (2006) Spine Ca 2 signaling in spike-timingdependent plasticity. J Neurosci 26:1100111013. Nunez A, Garca-Austt E, Buno W (1987) Intracellular -rhythm genera tion in identified hippocampal pyramids. Brain Res 416:289 300. Pelletier MR, Hablitz JJ (1994) Altered desensitization produces enhancement of EPSPs in neocortical neurons. J Neurophysiol 72:10321036. Raymond CR, Redman SJ (2002) Different calcium sources are narrowly tuned to the induction of different forms of LTP. J Neurophysiol 88:249 255. Raymond CR, Redman SJ (2006) Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus J Physiol (Lond) 570:97111. Regehr WG, Tank DW (1990) Postsynaptic NMDA receptor-mediated calcium accumulation in hippocampal CA1 pyramidal cell dendrites. Nature 345:807 810. Sah P, Bekkers JM (1996) Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. J Neurosci 16:4537 4542. Schiegg A, Gerstner W, Ritz R, van Hemmen JL (1995) Intracellular Ca2 stores can account for the time course of LTP induction: a model of Ca2 dynamics in dendritic spines. J Neurophysiol 74:1046 1055. Schiller J, Schiller Y, Clapham DE (1998) NMDA receptors amplify calcium influx into dendritic spines during associative pre- and postsynaptic activation. Nat Neurosci 1:114 118. Segal M, Auerbach JM (1997) Muscarinic receptors involved in hippocampal plasticity. Life Sci 60:10851091. Shouval HZ, Bear MF, Cooper LN (2002) A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci USA 99:1083110836. Sjostrom PJ, Turrigiano GG, Nelson SB (2001) Rate, timing, and cooperat ivity jointly determine cortical synaptic plasticity. Neuron 32:1149 1164. Song S, Miller KD, Abbott LF (2000) Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat Neurosci 3:919 926. Staubli U, Rogers G, Lynch G (1994) Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA 91:777781. Watanabe S, Hoffman DA, Migliore M, Johnston D (2002) Dendritic K channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 99:8366 8371. Yang H-W, Lin Y-W, Yen C-D, Min M-Y (2002) Change in bidirectional plasticity at CA1 synapses in hippocampal slices taken from 6-hydroxydopamine treated rats: the role of endogenous norepinephrine. Eur J Neurosci 16:11171128. Yasuda R, Sabatini BL, Svoboda K (2003) Plasticity of calcium channels in dendritic spines. Nat Neurosci 6:948 955. Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355 405.
You might also like
- Biochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967From EverandBiochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967D. RichterNo ratings yet
- TMP F204Document2 pagesTMP F204FrontiersNo ratings yet
- Baratta2002-Somatostatin LTP Dentate GyrusDocument9 pagesBaratta2002-Somatostatin LTP Dentate Gyrusiulia andreeaNo ratings yet
- RAT IN: Bicuculline Potential (EPSP) - Relationship EPSP Amplitude AmplitudeDocument14 pagesRAT IN: Bicuculline Potential (EPSP) - Relationship EPSP Amplitude AmplitudeFedrmNo ratings yet
- Lin Etal 2003Document9 pagesLin Etal 2003林瑜璿No ratings yet
- Cyclic AMP-mediated Inhibition of Noradrenaline-Induced Contraction Ca Influx in Guinea-Pig Vas DeferensDocument12 pagesCyclic AMP-mediated Inhibition of Noradrenaline-Induced Contraction Ca Influx in Guinea-Pig Vas DeferensssmorgNo ratings yet
- BF02462837Document4 pagesBF02462837ttqnhu.rhmNo ratings yet
- Wendy W. Wu, C. Savio Chan, D. James Surmeier and John F. DisterhoftDocument13 pagesWendy W. Wu, C. Savio Chan, D. James Surmeier and John F. DisterhoftFedrmNo ratings yet
- Muscarinic Receptors Modulate N-, P-, and L-Type Ca2+ Currents in Rat Striatal Neurons Through Parallel PathwaysDocument12 pagesMuscarinic Receptors Modulate N-, P-, and L-Type Ca2+ Currents in Rat Striatal Neurons Through Parallel PathwaysJunmajNo ratings yet
- Christian Ducreux and Jean-Jacques Puizillout - A-Current Modifies The Spike of C-Type Neurones in The Rabbit Nodose GanglionDocument13 pagesChristian Ducreux and Jean-Jacques Puizillout - A-Current Modifies The Spike of C-Type Neurones in The Rabbit Nodose GanglionFedrmNo ratings yet
- R.A. North and T. Tokimasa - Depression of Calcium-Dependent Potassium Conductance of Guinea-Pig Myenteric Neurones by Muscaric AgonistsDocument14 pagesR.A. North and T. Tokimasa - Depression of Calcium-Dependent Potassium Conductance of Guinea-Pig Myenteric Neurones by Muscaric AgonistsFedrmNo ratings yet
- Long-Term Potentiation of Intrinsic Excitability at The Mossy Fiber - Granule Cell Synapse of Rat CerebellumDocument9 pagesLong-Term Potentiation of Intrinsic Excitability at The Mossy Fiber - Granule Cell Synapse of Rat CerebellumJar JarNo ratings yet
- Soluble A Peptide Increases Excitatory Neurotransmission and Induces Epileptiform Activity in Hippocampal NeuronsDocument15 pagesSoluble A Peptide Increases Excitatory Neurotransmission and Induces Epileptiform Activity in Hippocampal NeuronsClaudia Alejandra Lopez ToroNo ratings yet
- Bear&KirkwoodDocument11 pagesBear&KirkwoodswagatarcNo ratings yet
- Effects of Low Concentrations of 4-Aminopyridine On CA1 Pyramidal Cells of The HippocampusDocument18 pagesEffects of Low Concentrations of 4-Aminopyridine On CA1 Pyramidal Cells of The HippocampusJortegloriaNo ratings yet
- T.G.J. Allen and G. Burnstock - Intracellular Studies of The Electrophysiological Properties of Cultured Intracardiac Neurones of The Guinea-PigDocument18 pagesT.G.J. Allen and G. Burnstock - Intracellular Studies of The Electrophysiological Properties of Cultured Intracardiac Neurones of The Guinea-PigLuammmNo ratings yet
- Delayed Increase of Acetylcholine Quantal Size Induced by THE Activity-Dependent Release of Endogenous CGRP But Not Atp in Neuromuscular JunctionsDocument25 pagesDelayed Increase of Acetylcholine Quantal Size Induced by THE Activity-Dependent Release of Endogenous CGRP But Not Atp in Neuromuscular JunctionsgarridotfNo ratings yet
- Biophysj00107 0239Document6 pagesBiophysj00107 0239aydinmemmedov9009No ratings yet
- Taniguchi 1983Document10 pagesTaniguchi 1983Giulia AndreeaNo ratings yet
- Membrane Stretch Affects Gating Modes of A Skeletal Muscle Sodium ChannelDocument17 pagesMembrane Stretch Affects Gating Modes of A Skeletal Muscle Sodium ChannelMichael MaierNo ratings yet
- Novel Strategy For Measuring Creatine Kinase Reaction Rate in The in Vivo HeartDocument10 pagesNovel Strategy For Measuring Creatine Kinase Reaction Rate in The in Vivo HeartYoung-Hoon SungNo ratings yet
- Lei 1998Document12 pagesLei 19980hitk0No ratings yet
- Burn Injury Decreases Myocardial Na-K-Atpase Activity: Role of PKC InhibitionDocument9 pagesBurn Injury Decreases Myocardial Na-K-Atpase Activity: Role of PKC InhibitionChristine Notoningtiyas SantosoNo ratings yet
- Rapid Temperature Jumps Using Infrared Diode Laser for Patch-Clamp StudiesDocument9 pagesRapid Temperature Jumps Using Infrared Diode Laser for Patch-Clamp StudiesAldo Alexander CamachoNo ratings yet
- TMP 75 AADocument9 pagesTMP 75 AAFrontiersNo ratings yet
- Jvms 83 997Document7 pagesJvms 83 997Blanca A SerranoNo ratings yet
- Takuya Yasui Et Al - Dynamic Synapses As Archives of Synaptic History: State-Dependent Redistribution of Synaptic Efficacy in The Rat Hippocampal CA1Document18 pagesTakuya Yasui Et Al - Dynamic Synapses As Archives of Synaptic History: State-Dependent Redistribution of Synaptic Efficacy in The Rat Hippocampal CA1FedrmNo ratings yet
- Activation of Medial Prefrontal Cortex by Phencyclidine Is Mediated Via A Hippocampo-Prefrontal PathwayDocument7 pagesActivation of Medial Prefrontal Cortex by Phencyclidine Is Mediated Via A Hippocampo-Prefrontal PathwayCortate15gNo ratings yet
- European Journal of PharmacologyDocument6 pagesEuropean Journal of Pharmacologyliska ramdanawatiNo ratings yet
- Adenylyl Cyclase in The Heart: An Enzymocytochemical and Immunocytochemical ApproachDocument6 pagesAdenylyl Cyclase in The Heart: An Enzymocytochemical and Immunocytochemical ApproachJoNo ratings yet
- Supporting Information: Roy Et Al. 10.1073/pnas.1708261114Document5 pagesSupporting Information: Roy Et Al. 10.1073/pnas.1708261114Jeffer ZevallosNo ratings yet
- Nitric Oxide, CGMP, and Hormone Regulation of Active Sodium TransportDocument6 pagesNitric Oxide, CGMP, and Hormone Regulation of Active Sodium TransportMichael Daley100% (1)
- Kondo 2000, Putative Ryanodine Receptors in The Sarcolemma of Ventricular Myocytes.Document7 pagesKondo 2000, Putative Ryanodine Receptors in The Sarcolemma of Ventricular Myocytes.Alessio LissoniNo ratings yet
- Brozoski 2005Document6 pagesBrozoski 2005Caesar Catalin CaratasuNo ratings yet
- TMP C32Document11 pagesTMP C32FrontiersNo ratings yet
- TMP 9 DA2Document11 pagesTMP 9 DA2FrontiersNo ratings yet
- Supplement Material Methods Cardiomyocyte IsolationDocument12 pagesSupplement Material Methods Cardiomyocyte IsolationhNo ratings yet
- Cordycepin - CA1 Membrane HyperpolarizationDocument5 pagesCordycepin - CA1 Membrane Hyperpolarizationttqnhu.rhmNo ratings yet
- Exercise Induces Muscle Fiber Type SwitchingDocument10 pagesExercise Induces Muscle Fiber Type SwitchingbookNo ratings yet
- BHP 311Document9 pagesBHP 311antoniorNo ratings yet
- A. Reyes Et Al - Passive Properties of Neostriatal Neurons During Potassium Conductance BlockadeDocument15 pagesA. Reyes Et Al - Passive Properties of Neostriatal Neurons During Potassium Conductance BlockadeFedrmNo ratings yet
- Plasticity in The Human Central Nervous SystemDocument15 pagesPlasticity in The Human Central Nervous SystemMarcelo LugonNo ratings yet
- Letter To The Editor: Doi: 10.3967/bes2017.125Document5 pagesLetter To The Editor: Doi: 10.3967/bes2017.125Ion CorbuNo ratings yet
- Control of Airway Smooth Muscle Tone. I - Electrophysiology and Contractile MediatorsDocument9 pagesControl of Airway Smooth Muscle Tone. I - Electrophysiology and Contractile MediatorsSergio Del AngelNo ratings yet
- Preclinical StudiesDocument16 pagesPreclinical StudiesFadhil Muhammad A.No ratings yet
- Hajos 2000Document11 pagesHajos 2000Breda MarcoNo ratings yet
- Physical Training Reverts Hippocampal Electrophysiological Changes in Rats Submitted To The Pilocarpine Model of EpilepsyDocument7 pagesPhysical Training Reverts Hippocampal Electrophysiological Changes in Rats Submitted To The Pilocarpine Model of EpilepsyJenivia LulileloNo ratings yet
- J Neurophysiol 2000 Xiong 3519 24Document7 pagesJ Neurophysiol 2000 Xiong 3519 24Renato MoraesNo ratings yet
- The Journal of Physiology - 2001 - Naves - Repetitive Nerve Stimulation Decreases The Acetylcholine Content of Quanta atDocument11 pagesThe Journal of Physiology - 2001 - Naves - Repetitive Nerve Stimulation Decreases The Acetylcholine Content of Quanta atAngelita SitumeangNo ratings yet
- Temporal Changes of Post Synaptic Signaling Molecules, Post Synaptic Density-95 and Neuronal Nitric Oxide Synthase, in The Inner Molecular Layer of The Mouse Dentate Gyrus During Voluntary RunningDocument8 pagesTemporal Changes of Post Synaptic Signaling Molecules, Post Synaptic Density-95 and Neuronal Nitric Oxide Synthase, in The Inner Molecular Layer of The Mouse Dentate Gyrus During Voluntary Runningsonjeonggyu87No ratings yet
- Cocaine and HipocampoDocument7 pagesCocaine and HipocampoNico TánatosNo ratings yet
- Dancing nano cell technical data science abh3602_smDocument65 pagesDancing nano cell technical data science abh3602_smbeetlebeeltehocoupleoftimesahNo ratings yet
- Distribution and Ultrastructure of Tyrosine Hydroxylasepositive Neurons in Cns of Bivalve Mollusc Under Action of Elevated Temperature and HypoxiaDocument2 pagesDistribution and Ultrastructure of Tyrosine Hydroxylasepositive Neurons in Cns of Bivalve Mollusc Under Action of Elevated Temperature and HypoxiaBea PippinNo ratings yet
- Gilden Et Al - 1995 - 1-f Noise in Human CognitionDocument4 pagesGilden Et Al - 1995 - 1-f Noise in Human CognitionAli Mert InalNo ratings yet
- Newly Formed Excitatory Pathways Provide A Substrate For Hyperexcitability in Experimental Temporal Lobe EpilepsyDocument12 pagesNewly Formed Excitatory Pathways Provide A Substrate For Hyperexcitability in Experimental Temporal Lobe EpilepsyHsieh Yun JuNo ratings yet
- Cunha-Reis BrJPharm143 (06) 733 (2004)Document12 pagesCunha-Reis BrJPharm143 (06) 733 (2004)dcrrufaNo ratings yet
- European Journal of PharmacologyDocument9 pagesEuropean Journal of PharmacologyMarcelo Pires de OliveiraNo ratings yet
- J. Biol. Chem.-2012-Zhang-33268-81Document15 pagesJ. Biol. Chem.-2012-Zhang-33268-81Soji AdimulaNo ratings yet
- Evaluation of Antiepileptic DrugsDocument32 pagesEvaluation of Antiepileptic DrugsRajpal SinghNo ratings yet
- Diets Prescribed in The Indian SystemDocument20 pagesDiets Prescribed in The Indian SystemBogdan BNo ratings yet
- Alejandra B Cardillo Et Al - Expression of Brugmansia Candida Hyoscyamine 6beta-Hydroxylase Gene in Saccharomyces Cerevisiae and Its Potential Use As BiocatalystDocument7 pagesAlejandra B Cardillo Et Al - Expression of Brugmansia Candida Hyoscyamine 6beta-Hydroxylase Gene in Saccharomyces Cerevisiae and Its Potential Use As BiocatalystFedrmNo ratings yet
- Y. V. Sheludko - Recent Advances in Plant Biotechnology and Genetic Engineering For Production of Secondary MetabolitesDocument9 pagesY. V. Sheludko - Recent Advances in Plant Biotechnology and Genetic Engineering For Production of Secondary MetabolitesFedrmNo ratings yet
- Zhenghao Qian Et Al - A Simple and Efficient Procedure To Enhance Artemisinin Content in Artemisia Annua L. by Seeding To Salinity StressDocument4 pagesZhenghao Qian Et Al - A Simple and Efficient Procedure To Enhance Artemisinin Content in Artemisia Annua L. by Seeding To Salinity StressFedrmNo ratings yet
- Nicholas P. Poolos - The H-Channel: A Potential Channelopathy in Epilepsy?Document6 pagesNicholas P. Poolos - The H-Channel: A Potential Channelopathy in Epilepsy?FedrmNo ratings yet
- Raoufa Abd El-Rahman Et Al - Production of Scopolamine and Hyoscyamine in Calli and Regenerate Cultures of Datura Metel (L.)Document9 pagesRaoufa Abd El-Rahman Et Al - Production of Scopolamine and Hyoscyamine in Calli and Regenerate Cultures of Datura Metel (L.)FedrmNo ratings yet
- Qingming Hou Et Al - Homeostatic Regulation of AMPA Receptor Expression at Single Hippocampal SynapsesDocument6 pagesQingming Hou Et Al - Homeostatic Regulation of AMPA Receptor Expression at Single Hippocampal SynapsesFedrmNo ratings yet
- Javier Palazón Et Al - Application of Metabolic Engineering To The Production of ScopolamineDocument21 pagesJavier Palazón Et Al - Application of Metabolic Engineering To The Production of ScopolamineFedrmNo ratings yet
- Nurussaba Khanam Et Al - Organogenesis, Differentiation and Histolocalization of Alkaloids in Cultured Tissues and Organs of Duboisia Myoporoides R. Br.Document8 pagesNurussaba Khanam Et Al - Organogenesis, Differentiation and Histolocalization of Alkaloids in Cultured Tissues and Organs of Duboisia Myoporoides R. Br.FedrmNo ratings yet
- Minyoung Shin and Dane M. Chetkovich - Activity-Dependent Regulation of H Channel Distribution in Hippocampal CA1 Pyramidal NeuronsDocument13 pagesMinyoung Shin and Dane M. Chetkovich - Activity-Dependent Regulation of H Channel Distribution in Hippocampal CA1 Pyramidal NeuronsFedrmNo ratings yet
- Suvi T. Hakkinen Et Al - Enhanced Secretion of Tropane Alkaloids in Nicotiana Tabacum Hairy Roots Expressing Heterologous Hyoscyamine-6beta-HydroxylaseDocument8 pagesSuvi T. Hakkinen Et Al - Enhanced Secretion of Tropane Alkaloids in Nicotiana Tabacum Hairy Roots Expressing Heterologous Hyoscyamine-6beta-HydroxylaseFedrmNo ratings yet
- Daniel Johnston and Rishikesh Narayanan - Active Dendrites: Colorful Wings of The Mysterious ButterfliesDocument9 pagesDaniel Johnston and Rishikesh Narayanan - Active Dendrites: Colorful Wings of The Mysterious ButterfliesFedrmNo ratings yet
- Rishikesh Narayanan and Daniel Johnston - The H Channel Mediates Location Dependence and Plasticity of Intrinsic Phase Response in Rat Hippocampal NeuronsDocument15 pagesRishikesh Narayanan and Daniel Johnston - The H Channel Mediates Location Dependence and Plasticity of Intrinsic Phase Response in Rat Hippocampal NeuronsFedrmNo ratings yet
- Mariela F. Perez, Francis J. White, and Xiu-Ti Hu - Dopamine D2 Receptor Modulation of K + Channel Activity Regulates Excitability of Nucleus Accumbens Neurons at Different Membrane PotentialsDocument13 pagesMariela F. Perez, Francis J. White, and Xiu-Ti Hu - Dopamine D2 Receptor Modulation of K + Channel Activity Regulates Excitability of Nucleus Accumbens Neurons at Different Membrane PotentialsFedrmNo ratings yet
- Lorenzo A. Cingolani et al- Activity-Dependent Regulation of Synaptic AMPA Receptor Composition and Abundance by β3 IntegrinsDocument30 pagesLorenzo A. Cingolani et al- Activity-Dependent Regulation of Synaptic AMPA Receptor Composition and Abundance by β3 IntegrinsFedrmNo ratings yet
- Martin Biel Et Al - Hyperpolarization-Activated Cation Channels: From Genes To FunctionDocument40 pagesMartin Biel Et Al - Hyperpolarization-Activated Cation Channels: From Genes To FunctionFedrmNo ratings yet
- Neil R. Hardingham Et Al - Presynaptic Efficacy Directs Normalization of Synaptic Strength in Layer 2/3 Rat Neocortex After Paired ActivityDocument12 pagesNeil R. Hardingham Et Al - Presynaptic Efficacy Directs Normalization of Synaptic Strength in Layer 2/3 Rat Neocortex After Paired ActivityFedrmNo ratings yet
- Severine Mahon Et Al - Role of A Striatal Slowly Inactivating Potassium Current in Short-Term Facilitation of Corticostriatal Inputs: A Computer Simulation StudyDocument7 pagesSeverine Mahon Et Al - Role of A Striatal Slowly Inactivating Potassium Current in Short-Term Facilitation of Corticostriatal Inputs: A Computer Simulation StudyFedrmNo ratings yet
- M. Diana Neely Et Al - Cortical Regulation of Dopamine Depletion-Induced Dendritic Spine Loss in Striatal Medium Spiny NeuronsDocument16 pagesM. Diana Neely Et Al - Cortical Regulation of Dopamine Depletion-Induced Dendritic Spine Loss in Striatal Medium Spiny NeuronsFedrmNo ratings yet
- Jason Aoto Et Al - Synaptic Signaling by All-Trans Retinoic Acid in Homeostatic Synaptic PlasticityDocument28 pagesJason Aoto Et Al - Synaptic Signaling by All-Trans Retinoic Acid in Homeostatic Synaptic PlasticityFedrmNo ratings yet
- Jay R. Gibson Et Al - Role For The Subthreshold Currents ILeak and IH in The Homeostatic Control of Excitability in Neocortical Somatostatin-Positive Inhibitory NeuronsDocument15 pagesJay R. Gibson Et Al - Role For The Subthreshold Currents ILeak and IH in The Homeostatic Control of Excitability in Neocortical Somatostatin-Positive Inhibitory NeuronsFedrmNo ratings yet
- Emilie Campanac Et Al - Downregulation of Dendritic Ih in CA1 Pyramidal Neurons After LTPDocument9 pagesEmilie Campanac Et Al - Downregulation of Dendritic Ih in CA1 Pyramidal Neurons After LTPFedrmNo ratings yet
- Claudia Clopath Et Al - Connectivity Reflects Coding: A Model of Voltage-Based Spike-Timing-Dependent-Plasticity With HomeostasisDocument27 pagesClaudia Clopath Et Al - Connectivity Reflects Coding: A Model of Voltage-Based Spike-Timing-Dependent-Plasticity With HomeostasisFedrmNo ratings yet
- Tiago Branco Et Al - Local Dendritic Activity Sets Release Probability at Hippocampal SynapsesDocument11 pagesTiago Branco Et Al - Local Dendritic Activity Sets Release Probability at Hippocampal SynapsesFedrmNo ratings yet
- Clifton C. Rumsey and L. F. Abbott - Synaptic Democracy in Active DendritesDocument13 pagesClifton C. Rumsey and L. F. Abbott - Synaptic Democracy in Active DendritesFedrmNo ratings yet
- Kathleen K. A. Cho Et Al - The Ratio of NR2A/B NMDA Receptor Subunits Determines The Qualities of Ocular Dominance Plasticity in Visual CortexDocument6 pagesKathleen K. A. Cho Et Al - The Ratio of NR2A/B NMDA Receptor Subunits Determines The Qualities of Ocular Dominance Plasticity in Visual CortexFedrmNo ratings yet
- Ithai Rabinowitch and Idan Segev - Two Opposing Plasticity Mechanisms Pulling A Single SynapseDocument7 pagesIthai Rabinowitch and Idan Segev - Two Opposing Plasticity Mechanisms Pulling A Single SynapseFedrmNo ratings yet
- Ingrid Van Welie Et Al - Different Levels of Ih Determine Distinct Temporal Integration in Bursting and Regular-Spiking Neurons in Rat SubiculumDocument12 pagesIngrid Van Welie Et Al - Different Levels of Ih Determine Distinct Temporal Integration in Bursting and Regular-Spiking Neurons in Rat SubiculumFedrmNo ratings yet
- Peter A. Appleby and Terry Elliott - Synaptic and Temporal Ensemble Interpretation of Spike-Timing-Dependent PlasticityDocument21 pagesPeter A. Appleby and Terry Elliott - Synaptic and Temporal Ensemble Interpretation of Spike-Timing-Dependent PlasticityFedrmNo ratings yet
- Jonathan E Rubin - Steady States in An Iterative Model For Multiplicative Spike-Timing-Dependent PlasticityDocument10 pagesJonathan E Rubin - Steady States in An Iterative Model For Multiplicative Spike-Timing-Dependent PlasticityFedrmNo ratings yet
- Ecg Stemi: Sequence of Changes in Evolving STEMIDocument3 pagesEcg Stemi: Sequence of Changes in Evolving STEMIFajar AgNo ratings yet
- C3 Carbon Fixation: Key Photosynthesis ProcessDocument8 pagesC3 Carbon Fixation: Key Photosynthesis ProcessBashiir NuurNo ratings yet
- Biology Assignment 2 Atefah Razack 1Document15 pagesBiology Assignment 2 Atefah Razack 1api-491258706No ratings yet
- What Is The Rate For Performing Chest Compressions For A Victim of Any Age A-30 Compressions Per Minute B - 50 Compressions Per Minute C - 80 Compressions Per MinuteDocument7 pagesWhat Is The Rate For Performing Chest Compressions For A Victim of Any Age A-30 Compressions Per Minute B - 50 Compressions Per Minute C - 80 Compressions Per MinuteHassan Shehri100% (2)
- Junctional: TachycardiaDocument11 pagesJunctional: TachycardiachaiNo ratings yet
- Respiratory Distress Syndrome (Hyaline Membrane Disease)Document98 pagesRespiratory Distress Syndrome (Hyaline Membrane Disease)Miraf MesfinNo ratings yet
- Calculation of q10 Value-1Document2 pagesCalculation of q10 Value-1Leighton ThompsonNo ratings yet
- Effects of A Balloon-Blowing Exercise On Lung Function of SmokersDocument4 pagesEffects of A Balloon-Blowing Exercise On Lung Function of SmokersMónica ReisNo ratings yet
- Lesson 1 - Introduction To AnemiaDocument10 pagesLesson 1 - Introduction To AnemiaThobby GamosaNo ratings yet
- Biology worksheet answersDocument2 pagesBiology worksheet answersAziyaNo ratings yet
- Human Body WebQuestDocument6 pagesHuman Body WebQuestjennyferchoNo ratings yet
- Q2 STEM General Biology 1 Week 2Document4 pagesQ2 STEM General Biology 1 Week 2MarkElijah ObelNo ratings yet
- PROTOCOL of Management of Critical CasesDocument131 pagesPROTOCOL of Management of Critical Casesaziz.shokry.mosaNo ratings yet
- Pulm 2005 Exam QuestionsDocument32 pagesPulm 2005 Exam QuestionsItharshan IndreswaranNo ratings yet
- Pediatric Critical Care Nutrition PDFDocument324 pagesPediatric Critical Care Nutrition PDFNia DefinisiNo ratings yet
- FINAL Lab Report #3Document10 pagesFINAL Lab Report #3Chippy RabeNo ratings yet
- TUGAS BAHASA INGGRIS 2 (Devi Yuliyanti)Document4 pagesTUGAS BAHASA INGGRIS 2 (Devi Yuliyanti)DeviNo ratings yet
- Blood ComponentsDocument2 pagesBlood ComponentsParul ShahNo ratings yet
- Lecture - 5 PDFDocument5 pagesLecture - 5 PDFDavid JokerNo ratings yet
- Anesthetic Considerations For Geriatric DogsDocument3 pagesAnesthetic Considerations For Geriatric DogsFernanda PérezNo ratings yet
- ABG Analysis: 6-Step ApproachDocument22 pagesABG Analysis: 6-Step Approachrajan40dmcNo ratings yet
- (Pharm) 1s-2 Ans DrugsDocument16 pages(Pharm) 1s-2 Ans DrugsKim Ramos67% (3)
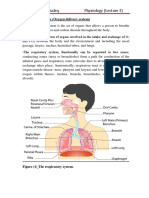
- Anatomy and Physiology of The Respiratory SystemDocument4 pagesAnatomy and Physiology of The Respiratory SystemchacameroNo ratings yet
- Pulmonary Function TestingDocument3 pagesPulmonary Function TestingAditya KumarNo ratings yet
- Pediatric Hematology, 3rd Ed. 2006Document2 pagesPediatric Hematology, 3rd Ed. 2006Henny HansengNo ratings yet
- The Utility of Non-Invasive Brain Stimulation in RDocument10 pagesThe Utility of Non-Invasive Brain Stimulation in RsanagaumiNo ratings yet
- Worksheet - Respiratory System PDFDocument3 pagesWorksheet - Respiratory System PDFDezta Fandity' VantycaNo ratings yet
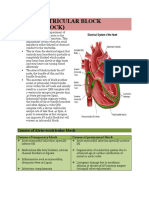
- Atrioventricular Block (Heart Block) : Causes of Atrio-Ventricular BlockDocument10 pagesAtrioventricular Block (Heart Block) : Causes of Atrio-Ventricular BlockLiza M. PurocNo ratings yet
- Laboratory Manual For Human Anatomy and Physiology Main Version 4th Edition Martin Solutions ManualDocument11 pagesLaboratory Manual For Human Anatomy and Physiology Main Version 4th Edition Martin Solutions ManualBradSalazarogzn100% (16)
- Drug Study: Medication Indication Contraindicati0N Side Effects Use Caution inDocument17 pagesDrug Study: Medication Indication Contraindicati0N Side Effects Use Caution inAngely Dianne Santiago II100% (2)
- Tales from Both Sides of the Brain: A Life in NeuroscienceFrom EverandTales from Both Sides of the Brain: A Life in NeuroscienceRating: 3 out of 5 stars3/5 (18)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4 out of 5 stars4/5 (3)
- All That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesFrom EverandAll That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesRating: 4.5 out of 5 stars4.5/5 (397)
- A Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsFrom EverandA Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsRating: 4.5 out of 5 stars4.5/5 (4)
- The Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceFrom EverandThe Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceRating: 4.5 out of 5 stars4.5/5 (515)
- 10% Human: How Your Body's Microbes Hold the Key to Health and HappinessFrom Everand10% Human: How Your Body's Microbes Hold the Key to Health and HappinessRating: 4 out of 5 stars4/5 (33)
- Gut: the new and revised Sunday Times bestsellerFrom EverandGut: the new and revised Sunday Times bestsellerRating: 4 out of 5 stars4/5 (392)
- Inside of a Dog: What Dogs See, Smell, and KnowFrom EverandInside of a Dog: What Dogs See, Smell, and KnowRating: 4 out of 5 stars4/5 (390)
- The Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionFrom EverandThe Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionRating: 4 out of 5 stars4/5 (811)
- Gut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)From EverandGut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)Rating: 4 out of 5 stars4/5 (378)
- A Series of Fortunate Events: Chance and the Making of the Planet, Life, and YouFrom EverandA Series of Fortunate Events: Chance and the Making of the Planet, Life, and YouRating: 4.5 out of 5 stars4.5/5 (62)
- Wayfinding: The Science and Mystery of How Humans Navigate the WorldFrom EverandWayfinding: The Science and Mystery of How Humans Navigate the WorldRating: 4.5 out of 5 stars4.5/5 (18)
- Who's in Charge?: Free Will and the Science of the BrainFrom EverandWho's in Charge?: Free Will and the Science of the BrainRating: 4 out of 5 stars4/5 (65)
- Moral Tribes: Emotion, Reason, and the Gap Between Us and ThemFrom EverandMoral Tribes: Emotion, Reason, and the Gap Between Us and ThemRating: 4.5 out of 5 stars4.5/5 (115)
- Fast Asleep: Improve Brain Function, Lose Weight, Boost Your Mood, Reduce Stress, and Become a Better SleeperFrom EverandFast Asleep: Improve Brain Function, Lose Weight, Boost Your Mood, Reduce Stress, and Become a Better SleeperRating: 4.5 out of 5 stars4.5/5 (15)
- The Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorFrom EverandThe Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorNo ratings yet
- Undeniable: How Biology Confirms Our Intuition That Life Is DesignedFrom EverandUndeniable: How Biology Confirms Our Intuition That Life Is DesignedRating: 4 out of 5 stars4/5 (11)
- Buddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomFrom EverandBuddha's Brain: The Practical Neuroscience of Happiness, Love & WisdomRating: 4 out of 5 stars4/5 (215)
- Crypt: Life, Death and Disease in the Middle Ages and BeyondFrom EverandCrypt: Life, Death and Disease in the Middle Ages and BeyondRating: 4 out of 5 stars4/5 (4)
- Human: The Science Behind What Makes Your Brain UniqueFrom EverandHuman: The Science Behind What Makes Your Brain UniqueRating: 3.5 out of 5 stars3.5/5 (38)
- The Lives of Bees: The Untold Story of the Honey Bee in the WildFrom EverandThe Lives of Bees: The Untold Story of the Honey Bee in the WildRating: 4.5 out of 5 stars4.5/5 (44)
- Good Without God: What a Billion Nonreligious People Do BelieveFrom EverandGood Without God: What a Billion Nonreligious People Do BelieveRating: 4 out of 5 stars4/5 (66)
- The Dog Who Couldn't Stop Loving: How Dogs Have Captured Our Hearts for Thousands of YearsFrom EverandThe Dog Who Couldn't Stop Loving: How Dogs Have Captured Our Hearts for Thousands of YearsNo ratings yet