0% found this document useful (0 votes)
879 views2 pagesOECD 423 - Acute Oral Toxicity Study: 6. Describe Details of Study Plan To Justify The Use of Animals
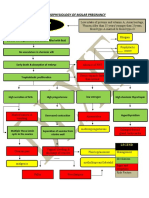
The document outlines an Acute Oral Toxicity Study (OECD 423) involving Wistar female rats to determine the acute oral toxicity of a test item and classify it according to GHS criteria. The study will be initiated after IAEC approval and is expected to be completed within one year, following a fixed dose procedure with a stepwise dosing approach. Observations will include mortality and signs of toxicity over a 14-day period, with all surviving animals undergoing necropsy at the end of the study.
Uploaded by
68Rajiv TejaniCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
879 views2 pagesOECD 423 - Acute Oral Toxicity Study: 6. Describe Details of Study Plan To Justify The Use of Animals
The document outlines an Acute Oral Toxicity Study (OECD 423) involving Wistar female rats to determine the acute oral toxicity of a test item and classify it according to GHS criteria. The study will be initiated after IAEC approval and is expected to be completed within one year, following a fixed dose procedure with a stepwise dosing approach. Observations will include mortality and signs of toxicity over a 14-day period, with all surviving animals undergoing necropsy at the end of the study.
Uploaded by
68Rajiv TejaniCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as DOCX, PDF, TXT or read online on Scribd