0% found this document useful (0 votes)
22 views1 pageChem 2
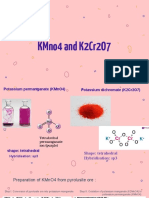
The document discusses the properties and reactions of potassium dichromate and potassium permanganate, highlighting their roles as oxidizing agents in organic chemistry. It details the interconversion of chromate and dichromate ions depending on the pH of the solution and provides specific half-reactions for various oxidation processes. Additionally, it outlines the preparation methods for potassium permanganate and its chemical behavior in solution.
Uploaded by
nf8tgcmd5nCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
22 views1 pageChem 2
The document discusses the properties and reactions of potassium dichromate and potassium permanganate, highlighting their roles as oxidizing agents in organic chemistry. It details the interconversion of chromate and dichromate ions depending on the pH of the solution and provides specific half-reactions for various oxidation processes. Additionally, it outlines the preparation methods for potassium permanganate and its chemical behavior in solution.
Uploaded by
nf8tgcmd5nCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd